1. Background
Beneficial health properties of fruits and vegetables have been emphasized by epidemiological studies (1). It is estimated that about 60% of the total world population use herbs and natural products in the treatment of some diseases (2). Phenolic compounds and fatty acids are two subclasses of natural products ubiquitous in all plant organs; therefore, are an integral part of the human diet. The content of total lipid and fatty acids composition are two key indices used for assessment of nutritional value of food (3). These compounds have beneficial applications in nutritional and health products and have been used for addressing various fundamental and pragmatic research problems in experimental biochemical, physiological and clinical studies (4). Polyunsaturated fatty acids (PUFAs), especially omega-3 have anti-inflammatory, antithrombotic, antiarrhythmic, hypolipidemic and vasodilator properties (5). Phenolic compounds have been studied for taxonomic purposes or to determine adulteration of food products (6). Two main types of polyphenols are flavonoids and tannins, which are powerful antioxidants in vitro. This property of phenolic compounds is due to absorption and neutralization of free radicals, quenching singlet and triplet oxygen and decomposing peroxides that may be related to health benefits against cardiovascular disease or cancer (7, 8). Flavonoids have been reported to have several biological effects including anti-carcinogenic, vasodilator, antimicrobial, hypolipidemic and hypoglycemic properties (9-12). Such benefits are reported frequently by experimental studies on animals or cultured human cell lines (13). Especially, these compounds are inhibitors of α-amylase, α-glucosidase and aldose reductase. α-amylase and α-glucosidase are two important enzymes involved in the regulation of glucose homeostasis (14). Aldose reductase catalyzes the reduction of excess glucose in various tissues (nerves, retina, lens and kidney) into sorbitol and plays a role of key enzyme in the polyol pathway. Thus, plants rich in phenolic compounds could be promising candidates for herbal management of diabetes (15).
2. Objectives
The aim of this research was to evaluate fatty acid compositions, total phenolic compounds, tannin and flavonoids of different extracts from roots and aerial parts of S. Paradoxa, which is a member of Asteraceae family. We also evaluated its antioxidant activity.
3. Materials and Methods
3.1. Chemicals
Folin-ciocalteu’s (FC) phenol reagent, sodium carbonate anhydrous, polyvinylpyrrolidone (PVP), acetic acid, heptadecanoic acid, sodium hydroxide, hydrochloric acid, potassium carbonate, aluminum chloride hexahydrate, sulphuric acid, standard fatty acid methyl esters and tannic acid were purchased from Merck company. Rutin and DPPH were purchased from Sigma chemical company (the United States).
3.2. Plant Material
Leaves and roots of S. Paradoxa were collected in April 2011 from south Khorasan province, Iran. The plant was identified by agriculture and natural research center of Khorasan in Mashhad. The plant tissues were separated, washed, dried in air and ground in a mixer.
3.3. Analysis of Fatty Acid Compositions
3.3.1. Methylation of Fatty Acids
Transesterification of FA was performed on lipid extract according to Christie with some modifications (16). Tissues (20 grams) were treated with 200 mL n-hexane for two hours. The extract was filtered and solvent was evaporated under reduced pressure using rotary vacuum evaporator. Oil (50 µL) treated with 8 mL of n-hexane/isopropanol (3:2) followed by addition of 1 mL of internal standard (0.5% of heptadecanoic acid in n-hexane). The mixture was incubated for 20 minutes in a water bath at 50°C and centrifuged for 10 minutes at 2000 rpm. The supernatant was separated and mixed with 6 mL distilled water. The organic phase (supernatant) evaporated by blowing N2 and 2 mL of 0.02 M methanolic NaOH was added to the mixture. The mixture was again incubated for 10 minutes at 50ºC. After cooling at room temperature, 2 mL of 2% HCl was added and the mixture was incubated for 10 minutes at 70ºC. The mixture was cooled at room temperature. Then, the solution was vortexed and centrifuged for 10 minutes at 2000 rpm followed by addition of 1 mL of n-hexane and 6 mL of 7% K2CO3. Fatty acid methyl esters were analyzed by GC-FID.
3.3.2. GC-FID Analysis
The GC-FID analysis was performed on a Varian 3800 instrument equipped with CP-Sil 88 column (100 m × 0.25 mm × 0.25 µm). Helium was applied as a carrier gas at a flow rate of 1.0 mL/min. Initial oven temperature was 180°C, increased to 195°C at 2°C/min and held at this temperature for two minutes. Then increased to 220°C at 2°C/min, where it was held for five minutes. The injection temperature was 250°C and Detector temperature was 270°C. Each sample (0.5 μL) was injected in split mode in the split ratio of 5:1. Identification of the fatty acid methyl esters was performed by a comparison of their retention times with standard fatty acid methyl esters. Quantification of the fatty acid methyl esters profile was performed considering the relative areas of peaks, expressed as the relative percentage of the individual area of each one versus the total area of compounds in the chromatogram.
3.4. Phenolic Content Analysis
3.4.1. General
The powdered material (50 g) was extracted with 70% ethanol during 48 hours. The extract was filtered and EtOH was evaporated. The aqueous phase was successively extracted with four solvents of increasing polarity (Et2O, CHCl3, EtOAc and n-BuOH).The extraction was continued to obtain colorless extract. All five extracts were evaporated to dryness and then dissolved in 50% ethanol to make 1% (w/v) solutions.
3.4.2. Determination of Total Phenolic Compounds (TPCs)
TPC of each extract was determined using the method of Ragazzi with modifications, using tannic acid as a standard (17). The mixture of sample extract (100 µL, 1%), FC reagent (2.5 mL, 0.5 M) and sodium carbonate (2 mL, 10%) were incubated for 60 minutes in the dark at ambient temperature. The absorbance was recorded at 760 nm on a UV-Vis spectrophotometer, using distilled water as the blank. The concentrations of TPC were expressed in milligram tannic acid equivalents (TAEs) per gram of dried tissues. All samples were analyzed in triplicates.
3.4.3. Analysis of Flavonoid Content
Total flavonoid content in the extracts was determined spectrophotometrically according to the method of Lozienė (18). Briefly, sample extract (1 mL) mixed with AlCl3.6H2O (1 mL, 2%) into a 25 mL volumetric flask and made up with 50% ethanol. The absorbance was read after 40 minutes at 415 nm. Blank samples were prepared from the mixture of 1 mL of plant extract and 1 drop of diluted acetic acid. The concentrations of flavonoid content were expressed in milligram rutin equivalents (REs) per gram of dried tissues. All samples were analyzed in triplicates.
3.4.4. Analysis of Tannin Content
The total tannins were determined using the method of Makkar et al. with modifications (17). The process has three steps. Step 1: this step was described in section 3.4.2. Step 2: the mixture of extract (1 mL), distilled water (1 mL) and PVP (0.1 g) was shaken for 5 minutes, incubated for 10 minutes at 4ºC and centrifuged for 5 minutes at 3000 rpm to obtain clear extract. Total phenolic compounds without tannins were determined in the extract using the method of section 3.4.2. Tannic acid was used for preparation of standard solution. Tannins were measured with differences between total phenolic compounds before and after addition of PVP.
3.5. Free Radical Scavenging Activity on DPPH
Samples (50 mg) were weighed and dissolved in methanol (1.5 mL, 80%). The mixture sonicated for 45 minutes, incubated for 15 minutes in the dark at ambient temperature and centrifuged for 15 minutes at 14000 rpm to obtain clear extract. 0.5 mL of extract was added to 0.5 mL of 80% methanol, thus diluting the concentration of sample by twofold. Samples were serially diluted 1/4, 1/8, 1/16 and 1/32. The mixture of DPPH (3.9 mL, 0.12 M in 80% methanol) and various dilutions of the extract (75 μL) were incubated for 30 minutes in the dark at ambient temperature. The absorbance was recorded at 517 nm on a UV-Vis spectrophotometer. All samples were analyzed in triplicates. The inhibition of DPPH radicals by the samples was calculated as follows:

Where, Ac is the absorbance without extract and As is the absorbance with the extract.
4. Results
4.1. Fatty Acid (FA) Compositions of Samples
The fatty acid compositions of the extracted oils were measured on the GC-FID by response factor for each fatty acid, using external standard curves and their amounts were calculated as percent of total fatty acids. The results are reported in Table 1. Total fatty acid (TFA) contents of leaves and root were not significantly different (60.36 and 51.92 mg/g, respectively). The leaves were found to be rich in unsaturated fatty acids (UFAs), 72.13% of TFAs. The primary UFAs were oleic and arachidonic acids, accounting for about 33.45% and 25.56% of TFAs, respectively. Oils rich in oleic and linoleic acids are the most adaptable of all oils and are excellent edible oils (19). The amount of stearic acid was higher than palmitic acid in both tissues. (84% of total saturated fatty acids (SFAs) in the leaves and 41.29% in root). Palmitic acid raises serum cholesterol, while stearic acid does not (20). In root, the amount of UFAs and SFAs were almost identical. The maximum amount of UFAs and SFAs were linoleic acid (14.95% of UFAs in leaves) and stearic acid (55.82% of SFAs in root), respectively. As shown in the Table 1, root contained the highest amount of SFAs (48.74% of TFAs) that was significantly different from the leaves.
| Fatty Acids | Tissue | |
|---|---|---|
| Leaves | Root | |
| Lauric acid (C12:0) | 0.75 | 2.3 |
| Myristic acid (C14:0) | 0.93 | 2.86 |
| Myristoleic acid (C14:1) | 0.15 | 1.44 |
| Palmitic acid (C16:0) | 0.76 | 8.36 |
| Palmitoleic acid (C16:1) | 1.24 | 1.31 |
| Stearic acid (C18:0) | 14.13 | 10.45 |
| Oleic acid (C18:1) | 20.19 | 2.6 |
| CLA (Trans C18:2) | 0.31 | 0.56 |
| Linoleic acid (Cis C18:2) | 0.73 | 6.51 |
| Linolenic acid (Cis C18:3) | 0.74 | 4.51 |
| GLA (Trans C18:3) | 0.45 | 1.82 |
| Eicosanoic acid (C20:0) | 0.25 | 1.34 |
| Arachidonic acid (C20:4) | 15.43 | 2.88 |
| Eicosapentaenoic acid (C20:5) | 0.61 | 1.41 |
| Docosa tetraenoic acid (C22:4) | 0.46 | 1.2 |
| Docosa pentaenoic acid (C22:5) | 1.85 | 1.03 |
| Docosa hexanoic acid (C22:6) | 1.38 | 1.34 |
Fatty Acid Compositions of Samplesa
4.2. Determination of Total Phenolic, Flavonoid and Tannin Contents
Total Phenolic, Flavonoid and Tannin contents of the S. Paradoxa extracts (H2O, n-BuOH, EtOAc, Chloroform and Ether) were also investigated. As shown in Table 2, a significant amount of phenolic compounds was observed in the n-BuOH extract of leaves and H2O extract of root (11.86 ± 0.1 mg TAEs/g for leaves and 3.36 ± 0.28 mg TAEs/g for root). Furthermore, total flavonoid contents were determined in the n-BuOH and ether extracts of leaves and root, respectively (6.42 ± 0.04 mg REs/g for leaves and 0.22 ± 0.00 mg REs/g for root). Differences in the amount of total phenolic and flavonoid content between extracts can be explained by different number of secretary structures in various plant tissues (2). The highest quantity of total tannins was found in n-BuOH extract of leaves and H2O extract of root (9.39 ± 0.03 mg TAEs/g for leaves and 2.56 ± 0.11 mg TAEs/g for root). The lowest quantity of these compounds was found in the CHCl3 extracts.
| Compounds | H2O | n-BuOH | EtOAc | Chloroform | Ether |
|---|---|---|---|---|---|
| Leaves | |||||
| Total phenolic b | 6.29 ± 0.4 | 11.86 ± 0.1 | 2.87 ± 0.09 | 0.057 ± 0.01 | 0.77 ± 0.1 |
| Total flavonoids c | 2.22 ± 0.06 | 6.42 ± 0.04 | 1.36 ± 0.02 | 0.012 ± 0.00 | 0.62 ± 0.01 |
| Total tannins b | 5.24 ± 0.18 | 9.39 ± 0.03 | 2.74 ± 0.05 | 0.041 ± 0.00 | 0.751 ± 0.06 |
| Root | |||||
| Total phenolic c | 3.36 ± 0.28 | 1.48 ± 0.09 | 0.74 ± 0.02 | 0.068 ± 0.01 | 1.65 ± 0.00 |
| Total flavonoids c | 0.077 ± 0.01 | 0.15 ± 0.01 | 0.091 ± 0.00 | 0.005 ± 0.00 | 0.22 ± 0.00 |
| Total tannins b | 2.56 ± 0.11 | 0.97 ± 0.06 | 0.693 ± 0.01 | 0.045 ± 0.01 | 1.61 ± 0.01 |
The Amounts of Phenolic Compounds, Flavonoid and Tannins in the Extracts of Tissues (n = 3) a
4.3. DPPH radical Scavenging Activity
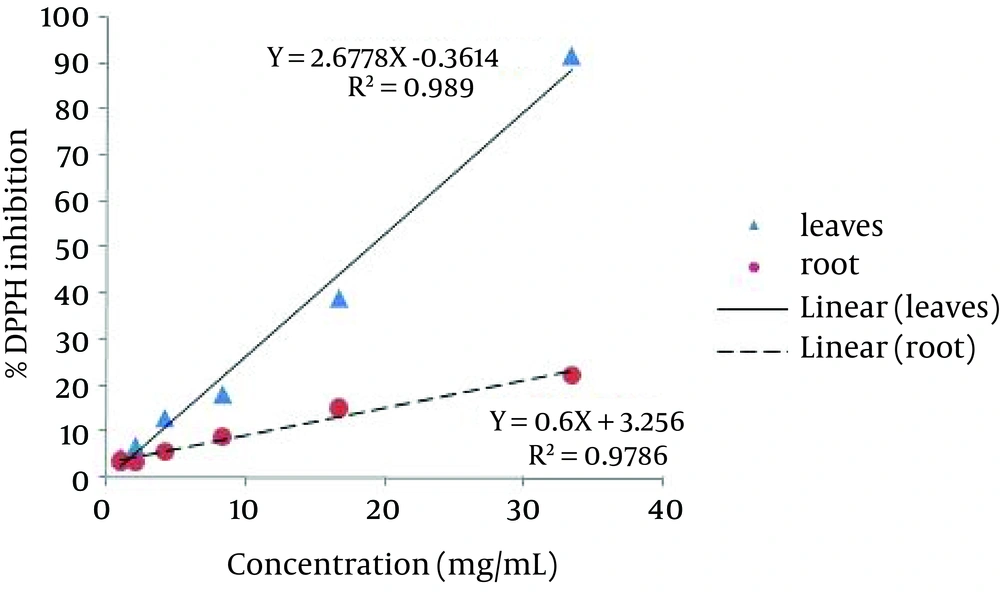
Radical scavenging activity of the extracts was determined using DPPH radical scavenging activity assay. Antioxidant activity of plant extracts is due to the presence of antioxidant secondary metabolites, such as phenolics, volatile oils, carotenoids and vitamins (21). The correlation between % DPPH inhibition (Y) and concentration of leaves and root extracts (X) had a correlation coefficient of R2 = 0.989, (Y = 2.6778X - 0.3614) and R2 = 0.9786, (Y = 0.6X + 3.256), respectively (Figure 1). Root extract (IC50 = 88.9 mg/mL) showed lower DPPH scavenging activity compared with leaves extract (IC50 = 18.81 mg/mL). Strong DPPH scavenging activity of leaves extract was partly due to the presence of phenolic compounds. Therefore, the leaves of S. Paradoxa are strong radical scavenger and considered as good sources of natural antioxidants.
5. Discussion
Lipids and fatty acids play a significant role in membrane biochemistry and have a direct impact on membrane-mediated processes such as osmoregulation, nutrient assimilation and transport (22). In addition, polyunsaturated fatty acids (PUFAs) play important roles in the treatment and prevention of cardiovascular, autoimmune, nervous disease, inflammatory conditions, heart disease, atherosclerosis, autoimmune disorders, diabetes and other diseases, improvement of learning ability and process of lipid peroxidation (2, 3, 23). S. Paradoxa as a member of Asteraceae family and mainly found in the eastern region of Iran is widely used as vegetable. The leaves of S. Paradoxa were found to be rich in unsaturated fatty acids (UFAs), 72.13% of TFAs. The primary UFAs were oleic and arachidonic acids, accounting for about 33.45% and 25.56% of TFAs, respectively. Oils rich in oleic and linoleic acids are the most adaptable of all oils and are excellent edible oils (24). Linoleic acid shows anti-carcinogenic activity, protection against arteriosclerosis and reduction of body fat (25). Furthermore, family of Asteraceae has a very simple flavonoid pattern (26). A significant amount of phenolic and flavonoid compound (11.86 ± 0.1 mg TAEs/g and 6.42 ± 0.04 mg REs/g for leaves, respectively) in S. Paradoxa would play an important role in further development and use of this herb. Antioxidant and anti-diabetic properties of plant extracts are closely related to their phenolic compounds and these compounds greatly contribute to the organoleptic properties by affecting the color, astringency, aroma, favor, bitterness and oxidative stability of products (13, 27, 28). The DPPH scavenging activity was higher in the leaves extract of S. Paradoxa than root extract (IC50 = 18.81 and 88.9 mg/mL, respectively). In this study, antioxidant activity of S. Paradoxa was related with its amount of phenolic and flavonoid contents, which is consistent with previous reports (29, 30). Such a favorable composition of unsaturated fatty acids and flavonoid content of leaves suggests that this plant might have potential as a new raw material of food products for food industry. In addition, due to high phenolic content and high DPPH scavenging activity of the leaves, S. Paradoxa is a promising source of natural antioxidants and may have good antidiabetic activity.