1. Background
Rice from the Gramineae family with the scientific name “Oryza sativaLinn” is one of the most important staple foods in the world (1). In Asian countries, high amounts of rice are consumed annually per capita. According to the Food and Agriculture Organization of the United Nations (FAO), in 2008 the worldwide rice production was forecast to be 661.3 million tons. In addition, the annual consumption of rice, which showed a slight increase in 2008, is about 57.2 kg per capita and year (2). Rice is cultivated in different areas of Iran that have a sultry and rainy climate. Since the amount of rice cultivation is not enough, the country imports rice to meet the domestic demand from other regions such as India, Pakistan, Bangladesh, and Thailand, which are among the largest producers of rice in the World.
FAO estimated that at least 25% of the world’s cereal grains are contaminated by mycotoxins, including aflatoxins (AFs) (3). AFs are potent toxic, carcinogenic, mutagenic, immunosuppressive agents, produced as secondary metabolites by the fungi Aspergillus flavus and Aspergillusparasiticus in a variety of important agricultural products worldwide, e.g. corn, wheat, rice, spices, dried fruits, and nuts, under favorable temperature and humidity (4-6). Among all four major AFs, which are AFB1, AFB2, AFG1 and AFG2, AFB1 is one of the most potent environmental mutagens and carcinogens known (7, 8).
The International Agency for Research on Cancer (IARC) has classified AFB1 as a Group I carcinogen, primarily affecting the liver (9).
The Institute of Standards and Industrial Research of Iran (ISIRI) has set a maximum tolerable level (MTL) for AFB1 and total AFs of 5 and 30 ng/g, respectively (10).
Ahvaz is the capital city of Khuzestan Province and is located in the southwest region of Iran, sharing a border with Iraq’s Basra Province and the Persian Gulf. Other than geopolitical importance, Ahvaz plays a vital role in the economy of Iran and is one of the main cities of Iran for importing commodities such as rice. The hot and humid climate in Ahvaz is suitable for the growth of AFs. There is a paucity of data on the level of AFs in the imported rice in this area. It is, therefore, necessary to determine the levels of AFs in rice, which is one of the most popular staple foods in this region.
2. Objectives
This study was carried out to determine the concentration of AFs in the imported rice in the markets of Ahvaz city, Iran.
3. Materials and Methods
3.1. Chemicals, Reagents and Materials
All solvents used for the experiments (i.e. methanol, acetonitrile, and deionized water) were High-Performance Liquid Chromatography (HPLC) grade. Biopure AFs mix standard solutions containing B1, B2, G1, and G2 were obtained from Sigma (St. Louis, USA) at concentrations of 2.020, 0.494, 2.010, and 0.495 µg/mL, correspondingly. AFs working solutions were prepared by dilution in the same solvent. AflaTest immunoaffinity columns were purchased from Romer Company.
3.2. Samples
Ninety samples, comprising 30 samples of each of three brands (1, 2, and 3) of imported rice, were purchased from different supermarkets in Ahvaz and transferred to the Toxicology Lab of the Department of Toxicology and Pharmacology, Pharmacy School of Ahvaz Jundishapur University of Medical Sciences. All the samples were stored at proper temperature and condition until analysis.
3.3. Apparatus
The Shimadzu 10ADvp HPLC System (Japan) was equipped with a Shimadzu RF-10AXL Fluorescence Detector, Shimadzu LC-10ADvp Pump, isocratic mode, Shimadzu DGU-14A Degasser, Shimadzu SCL-10Avp System Controller, Shimadzu FCL-10ALvp Flow Controller, and Shimadzu LC Solution Software. The column (4.6 × 250 mm), which was packed with particles of silica modified with octadecylsilyl groups (4 µm in diameter), was purchased from Capital Co., England.
3.4. Clean-up by Immunoaffinity Column Chromatography
First, 5 grams of NaCl and 100 mL of CH3OH:H2O (80:20) was added to 50 grams of each milled sample and blended for 3 minutes. Then, it was filtrated through a prefolded filter. Next, 10 mL of the filtrate was diluted with 40 mL of deionized water. The diluted solution was filtered through microfiber paper and 10 mL of that was applied to the immunoaffinity column (AflaTest column) specific for AFB1, AFB2, AFG1, and AFG2. In the column, AFs bonded to antibodies. After concentration and purification with water, the AFs were removed from the antibodies with 1 mL of CH3OH and diluted with 1 mL of H2O. Finally, 200 µL of this solution was injected into the HPLC.
3.5. Quantitative Analysis
Each AFs peak in the chromatograms was identified by comparing its retention time with that of the corresponding reference standard quantified by the HPLC (C18, 250 × 4.6 mm: 4 μm, 360 nm excitation, 440 nm emission with fluorescence detection and post-column bromide derivatization method, mobile phase of water-acetonitrile-methanol (600:200:200 v/v) +119 mg potassium bromide +100 µL con. HNO3 at a flow rate of 1 mL/min). Calibration curves were drawn at concentrations of 0.5, 1, and 2 ng/mL for AFB1; 0.123, 0.246, and 0.492 ng/mL for AFB2; 0.497, 0.994, and 1.980 ng/mL for AFG1; and 0.123, 0.246, and 0.492 ng/mL for AFG2.
The concentrations of B1, B2, G1, and G2 in the rice samples were calculated by using the equation of the calibration curve of each species. Recovery was performed by adding 2 mL of each of 0.5, 1, and 2 ng/mL of the standard solutions of AFB1 (6 repeats for each level) into 10-mL volumetric flasks and evaporating it under nitrogen gas. The residues in the volumetric flasks were diluted to the mark by adding the required volume of one of the rice samples filtrate whose AFs content had been determined. Then, the procedure for clean-up was followed as above.
3.6. Statistical Analysis
The concentration of total AFs (AFB1, AFB2, AFG1, and AFG2) was calculated as follows:
Total AFs = Concentration of AFB1 + AFB2 + AFG1 + AFG2
The data were expressed as mean ± standard deviation and were analyzed with Statistical Package for the Social Sciences (SPSS 20) software. The differences between the mean concentrations of AFs in the different brands of rice were calculated using the One-Way Analysis of Variance (ANOVA) and the Tukey post-hoc test for the significant interrelation between the brands of rice. The difference between the mean concentrations of AFB1 and total AFs in the three brands with the MTL were statistically significant. A P value < 0.05 was considered statistically significant.
4. Results
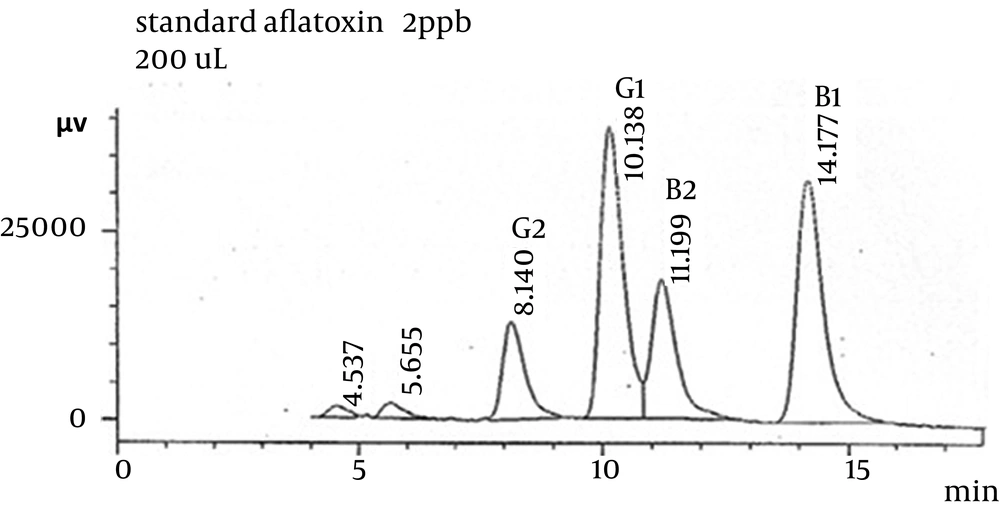
The retention times for AFB1, AFB2, AFG1, and AFG2 under this condition were 14.177, 11.199, 10.138, and 8.140 min, respectively (Figure 1).
The average recoveries and relative standard deviation (RSD %) for the intraday and interday of the applied analytical methods for AFB1, AFB2, AFG1, and AFG2 in the rice are shown in Table 1. All the recoveries were more than 89%, indicating the high accuracy of the method. The limits of detection and quantification for AFs were determined (Table 2). The highest concentration of AFB1 and total AFs in the investigated rice samples were 2.3500 and 2.7040 ng/g, respectively. The different mean concentration of AFB1 and total AFs in the three brands of rice samples were significantly lower than the MTL of AFB1 (5 ng/g) and total AFs (30 ng/g) set by the ISIRI (P < 0.001). There were no significant differences between the mean values of AFB1 and total AFs in the three different brands of the rice samples.
| Aflatoxins | Spiking Levels, ng/mL | Recovery, % | Intraday, RSD% | Interday, RSD% |
|---|---|---|---|---|
| AFB1 | 2 | 98 | 1.7 | 6.49 |
| 1 | 100 | 1.8 | 6.8 | |
| 0.5 | 90 | 2.1 | 7.3 | |
| AFB2 | 0.492 | 104 | 3 | 7.2 |
| 0.246 | 97 | 3.4 | 7.4 | |
| 0.123 | 89 | 3.8 | 7.7 | |
| AFG1 | 1.98 | 98 | 4.3 | 8.7 |
| 0.994 | 96 | 4.8 | 8.9 | |
| 0.497 | 94 | 5.1 | 9.5 | |
| AfG2 | 0.490 | 106 | 6.3 | 12.3 |
| 0.245 | 98 | 6.7 | 12.7 | |
| 0.123 | 89 | 6.9 | 13.1 |
aAbbreviations: AF, Aflatoxin; RSD, Relative standard deviation.
| Aflatoxin | LOD, ng/mL | LOQ, ng/mL |
|---|---|---|
| AFB1 | 1.8 × 10-3 | 6.1 × 10-3 |
| AFB2 | 8.7 × 10-4 | 2.8 × 10-3 |
| AFG1 | 2.1 × 10-3 | 7.2 × 10-3 |
| AFG2 | 4.8 × 10-3 | 16 × 10-3 |
The mean concentration of Aflatoxins (AFs) (i.e. AFB1, AFB2, AFG1, AFG2, and total AFs) for each brand of the rice samples is depicted in Table 3.
| Brand of Rice | Number of Positive Samples | Concentration of Aflatoxins a |
|---|---|---|
| 1 | ||
| AFB1 | 17 | 0.2788 ± 0.7772 |
| AFB2 | 23 | 0.0165 ± 0.0351 |
| AFG1 | 17 | 0.0420 ± 0.0813 |
| AFG2 | 17 | 0.0436 ± 0.1224 |
| Total AFs | 23 | 0.3810 ± 0.8791 |
| 2 | ||
| AFB1 | 25 | 0.0619 ± 0.1157 |
| AFB2 | 25 | 0.0047 ± 0.0099 |
| AFG1 | 25 | 0.3244 ± 0.7435 |
| AFG2 | 25 | 0.0404 ± 0.0766 |
| Total AFs | 25 | 0.4314 ± 0.7462 |
| 3 | ||
| AFB1 | 30 | 0.0121 ± 0.0235 |
| AFB2 | 30 | 0.0009 ± 0.000 |
| AFG1 | 30 | 0.2042 ± 0.5273 |
| AFG2 | 30 | 0.0184 ± 0.0359 |
| Total AFs | 30 | 0.2357 ± 0.5286 |
a Data are presented as Mean ± SD.
5. Discussion
AFs are one of the most potent toxins that can affect human health (11, 12). Diet is one of the main routes by which humans are exposed to AFs and the possible adverse effects resulting from long-term exposure to low levels of AFs, which may lead to health problems (13). Given the importance of mycotoxins, the literature abounds with research reporting that food and feed items have been contaminated with mycotoxins such as AFB1, AFB2, AFG1, and AFG2 (14-20).
Approximately, 100 countries, including Iran, have developed MTL for mycotoxins in foodstuffs and feedstuffs. The MTL for AFB1 and total AFs in rice in Iran is 5 and 30 ng/g, respectively. The results of the present research indicated that the concentrations of AFB1 and total AFs in all of the investigated rice samples were below these limits. According to the results of this study, none of the investigated rice samples had contamination more than the MTL in Iran (Table 3). Mazaheri (14) analyzed 71 imported rice samples to Iran between March 2006 and March 2007 for AFs using the immunoaffinity column quantified by the HPLC. Among the 71 investigated rice samples, AFB1 was detected in 59 samples (83% of the total) at the mean concentration of 1.89 ng/g for all the samples (with the undetected samples taken as zero). Total AFs was detected in 59 samples (83% of the total) at a mean concentration of 2.09 ng/g for the samples. The AFB1 level in two (2.8%) samples was above the MTL of AFB1 in Iran (5 ng/g). Regarding total AFs, the mean contamination level (2.09 ng/g) was lower than the MTL of total AFs in rice in Iran and lower than the maximum level of the EU for total AFs (4 ng/g). Additionally, only nine samples had levels above the MTL of the EU in total AFs (14). Feizy et al. (16) determined AFs levels in 261 rice samples by using the HPLC after post-column derivatization with iodine by fluorescence detection. The results indicated that 68.9% of the rice samples contained AFB1 at levels > 0.2 ng/g (16).
Zaboli et al. (20) characterized isolated Aspergillus fungus in new and old rice bran samples and determined the correlation between AFB1 production and Aspergillus contamination. For this purpose, they collected 30 rice samples from different regions of Mazandaran Province, northern Iran. The averages of AFB1 in the new and old rice bran samples were found to be 5.07 and 6.81 µg/kg, respectively. No significant difference was observed in terms of the AFB1 value between the new and old samples. In addition, there were significant correlations between the culture results and the AFs production only in the old samples (P < 0.05) (20).
Rahmani et al. (17) measured AFs in 256 rice samples collected from retail markets in different provinces of Iran between October 2007 and July 2008 by using the HPLC with fluorescence detection and post-column derivatization. The levels of contamination ranged from 0.0 to 5.8 ng/g (mean = 1.4 ng/g) and 0.1 - 6.3 ng/g (mean = 1.6 ng/g) for AFB1 and total AFs, correspondingly (17).
Bansal et al. (18) analyzed 200 samples of rice (including white, brown, red, black, basmati, and jasmine, as well as wild rice) from the United States, Canada, Pakistan, India, and Thailand for AFs and other mycotoxins in two different years. According to the results of that study, the five most contaminated samples in each year contained 1.44 - 7.14 ng/g of AFB1 (year 1) and 1.45 - 3.48 ng/g of AFB1 (year 2); they were mostly basmati rice from India and Pakistan and black and red rice from Thailand (18). Mohammadi et al. (15) reported that among 152 imported rice samples to Bushehr in Iran, 35 (23.03%) samples did not have any total AFs contamination. Also, 76.97% of the samples were contaminated, ranging between 0.15 and 4.27 at a mean of 0.671 for total AFs and ranging between 0.09 and 3.3 at a mean of 0.46 ng/g for AFB1, respectively (15). The results of that study showed that the levels of AFB1 contamination in all the samples were less than the MTL of 5 ng/g, which chimes in with our results.
The estimated daily intake of total AFs from rice depends on both the AFs concentration in rice and the daily rice consumption. In addition, the body weight of the human can influence the tolerance of contaminants. The estimated daily mean total AFs intake from each rice product by a person is calculated as follows:
Estimated daily intake (EDI) of total AFs (ng AFs / kg bw / day) = (daily mean rice intake, g/day) × (mean total AFs concentration in the corresponding rice products, ng/g) / kg body weight
Sales and Yoshizawa (19) measured AFs levels in 78 polished and brown rice samples in the Philippines. The mean and range concentration of AFs for the positive polished and brown rice samples were 0.37 (< 0.025 - 2.7) and 2.7 (0.03 - 8.7 µg/kg), respectively. The estimated potential daily intake of AFB1 from rice is between 0.1 and 7.5 ng/kg of body weight/day, the mean of which is 1.0 ng representing 9.1 - 5.3 times the estimated tolerable daily intake for AFB1 reported to date for Asia (19). Based on a consumption survey in Iran, the average consumption of rice is 107 g per day per person (21). So, regardless of the reduction in the AFs concentration in rice during cooking, according to the results of this study, the estimated daily exposures to rice total AFs of the low, average, and high consumers were in the range of 0.1686 - 0.9244 ng /kg bw/day (Table 4). These were less than the reference value of 1 ng /kg bw/day (22-24). The mission of the national food control system of each country is to protect its citizens from the harmful effects that pollutant substances in food may cause. A control program for food-pollutant components from field to table should be based on the criteria of the Hazard Analysis and Critical Control Point (HACCP) approach, which will require an understanding of the important aspects of the interactions of the toxic materials with crop plants; the methods or technologies that can be applied either in the field (pre-harvest) or in drying, storage, and transportation (postharvest); the development of processed foods for human consumption; the production of livestock using feeds and processed feeds, including diagnostic capabilities for diseases; and an understanding of the marketing and trade channels, including storage and delivery of foods to the consumer's table.
| Consumer Groups | Amount of Rice Intake, g rice/day | Intake of Total AFs, ng/kg bw/day a | ||
|---|---|---|---|---|
| Brand 1, 0.3810, ng/g | Brand 2, 0.4314, ng/g | Brand 3, 0.2360, ng/g | ||
| Low | 50 | 0.2721 | 0.3081 | 0.1686 |
| Moderate | 107 | 0.5824 | 0.6594 | 0.3607 |
| High | 150 | 0.8164 | 0.9244 | 0.5057 |
a Mean Values for the Rice Samples.
Therefore, food control authorities should change technologies in food production, processing, and marketing; develop science-based food control systems with a focus on consumer protection; harmonize food safety and quality standards at international levels; and finally grow consumers’ awareness of food safety and quality issues. Accordingly, a good testing protocol for mycotoxins is necessary to manage all the control points so as to ultimately ensure a food supply free of toxic levels of mycotoxins for the consumer.