1. Background
Insomnia or sleeplessness is a disorder characterized by a personal incapability to falling or staying asleep for a desirable period of time, therefore disturb in quality of life (1). Different factors can cause insomnia; some of them are including illness, stress and noise (2). Insomnia disorder for long time led to poor memorizing, slower reactions, emotional disturbances, and changes in the immune response (3). Nowadays, different drugs e.g. benzodiazepines or non-benzodiazepines, antidepressants and antihistamines are used for insomnia disorder. Benzodiazepines and benzodiazepine-like compounds are the most widely used as hypnotics but they show many unpleasant reactions, such as drug dependence, tolerance, rebound insomnia and amnesia (4). As a result, for increasing of efficacy, they are forced to elevate dose (5). Therefore, studies for findings new hypnotic agents with lesser side effect and more effectiveness have continued. Herbal agents always have been a good source for developing new therapeutics for treatment of diseases (6-8). The genus of Nepeta with the common persian name of pune-sa includes 67 species that are found all over of Iran. Nepetaglomerulosa (lamiaceae) is one of species of this genus. It was found in Asia (widely distributed in Iran, especially areas of Khorasan and Isfahan provinces), America and Europe. The aerial parts of Nepeta glomerulosa are empirically used in folk medicine as antispasmodic, diuretic, antiseptic, antitussive, antiasthmatic, and febrifuge activities. Some nepeta species have been reported to have antinociceptive (N. italica; N. cataria), antimicrobial (N. crispa) and antiviral (N. cataria) effects (9-12). Ingredients of nepeta glomerulosa extract are alkaloid, saponin and oils (13). It’s oil contains over 35 components. The major components were α-pinene (18.3%), 1,8-cineole (13.9%), limonene (9.7%), gernyl acetate (9.3%), caryophyllene oxide (8.0%), linalool (4.8%), trans-β-ocimene (4.7%), humulene epoxide (4.2%), trans-α-bergamotene (3.5%), α-humulene (3.2%) and camphene (3.1%) (14, 15). Also, Hosseinzadeh and co-workers showed that aqueous extract of nepetaglomerulosa reduces morphine withdrawal syndrome (16).
2. Objectives
Because of some species of Nepeta such as Nepeta cataria and Nepeta menthoides (17) increased sleeping time, therefore the present study was designed to evaluate sleep-prolonging effect of Nepeta glomerulosa hydro-alcoholic extract (HAE) and its fractions. Also, the safety of this plant was examined using neuronal cells.
3. Materials and Methods
3.1. Drugs and Chemicals
Pentobarbital sodium, penicillin-streptomycin and flumazenil were bought from Sigma (USA). Diazepam was obtained from Chemidarou Company (Iran). Tween 80 was from Merck (Germany). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from GIBCO (USA). Flumazenil, pentobarbital and diazepam were dissolved in saline to make a 30 mg/mL and 3 mg/mL solutions, respectively.
3.2. Plant Collection and Extraction
3.2.1 Plant Material
The aerial parts of Nepetaglomerulosa Boiss.were collected from Torbat Jam Bazd mountains (26.5.2005, at an altitude of 1850 m). The plant was identified by Mr. Ahi, and voucher samples (152-1407-2) are preserved for reference at the herbarium of the department of pharmacognosy, faculty of pharmacy, Mashhad University of Medical Sciences. The HAE were prepared with maceration method. The powder of aerial parts of plant (100 g) was macerated in 1000 mL of 70% ethanol for 48 hours (18). The extract was then filtered and dried on a water bath. The yield of the dried extract related to the weight of the dried aerial parts was 21.04 g.
For preparation of the extract fractions, 10 g of dried HAE was suspended in 200 mL distilled water and transferred to a separator funnel. Using solvent-solvent extraction, it was fractionated with ethyl acetate or N-butanol. The EAF and n-butanol fraction (NBF) were separated to obtain water fraction (WF) (19). All the fractions were dried on a water bath and working solutions made up in saline (WF) or distilled water containing 10% DMSO (EAF and NBF). The yield of WF, NBF and EAF was respectively, 5 g, 3.16 and 1.7.
3.3. Animals
Male albino mice weighting 20 - 30 g were maintained at a controlled temperature (22 ± 1°C) with a 12 hours light and dark cycle and free access to water and food. The study was carried out in accordance with ethical guidelines of Mashhad University of Medical Sciences. The animals were randomly divided into 12 groups, each one consisting of 8 mice. At the beginning, hypnotic effect of HEA was evaluated in seven groups: saline as vehicle, diazepam (3 mg/Kg) as positive control and HAE (50, 100, 150 and 200 mg/Kg). Also, in three groups of animals, hypnotic effect of HAE-fractions including WF (100 mg/kg), EAF (100 mg/kg) and NBF (50 and 100 mg/kg) were investigated. Moreover, flumazenil was applied (2 mg/kg) as diazepam antagonist for identifying mechanism.
3.4. Evaluation of Pentobarbital-Induced Sleep
The hypnotic assessment method was based on prolongation of sleep induced by pentobarbital (20). Briefly, a single dose of HAE (50, 100, 150 and 200 mg/kg), fractions of HAE, diazepam (3 mg/kg), or vehicles was injected (i.p.) to the mice. After 30 minutes, pentobarbital (30 mg/kg, i.p.) was administrated to induce sleep. Flumazenil (2 mg/kg) was administrated 30 minutes before diazepam or HAE. The animals were considered asleep if stayed immobile and lost their righting reflex when positioned on its back. The time interval between administration of pentobarbital and onset of sleep was considered as sleep latency.
3.5. LD50 Determination
Eight groups, each containing 2 mice were used for determination of LD50 of HAE. Groups 1 - 7 were injected intraperitoneally with 50, 100, 200, 400, 800, 1600 and 3200 mg/kg of HAE and group 8 received normal saline as vehicle. Mortality rate was observed and recorded for 24 hours period. The highest dose which did not kill any mice and the lowest dose which led to death of one animal were recorded. The mean of these two doses was considered as the median lethal dose (18, 21).
3.6. Neurotoxicity Assessment
The possible cytotoxicity of Nepeta glomerulosa was tested on rat pheochromocytoma-derived cells (PC12). The cells were cultured in 96-well plates for 24 hours in DMEM supplemented with 10% FBS, penicillin (100 units/mL) and streptomycin (100 µg/mL). Then, the culture medium was changed to fresh one containing vehicle (DMSO 1%) or HAE (50, 100, 150, 200, 400 and 800 µg/mL). The cells were incubated for 24 hours at 37 ˚C in an atmosphere of 5% CO2. Then, cell proliferation was evaluated using MTT assay as previously described (20, 22, 23). Briefly, the MTT solution was added to culture medium to make final concentration of 0.5 mg/ml and incubated for 2 hours. Then, the medium was discarded and the resulting formazan day dissolved with DMSO. The optical density of dye was measured at 545 nm.
3.7. Statistics
All values are expressed as mean ± SEM. statistical analysis was performed using one way analysis of variance (ANOVA) followed by Tamhane, s T2 post hoc test by Instat software package. Differences were considered significant at P < 0.05.
4. Results
4.1. Effects of nepeta glomerulosa on Duration of Sleep
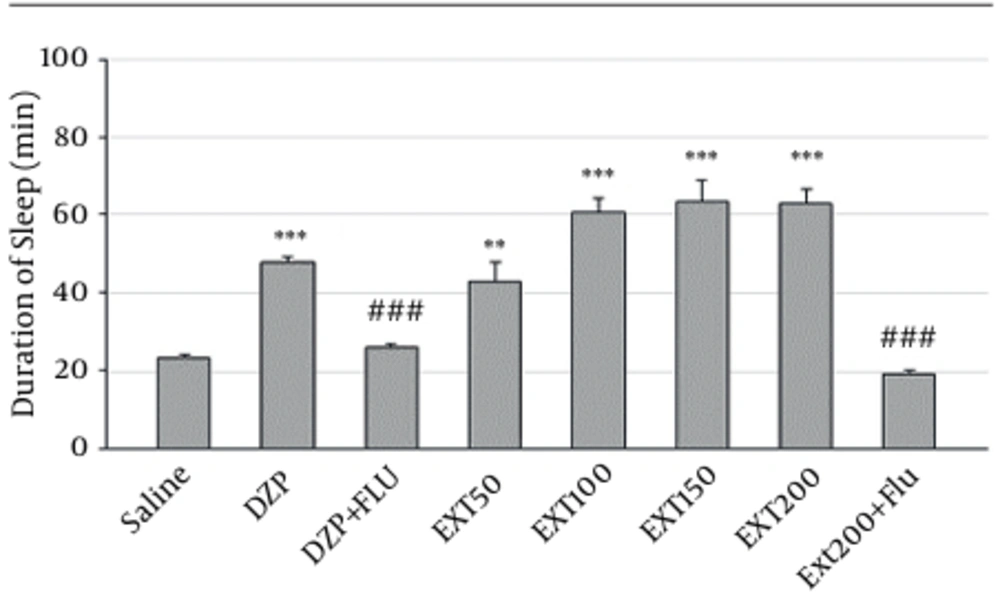
Sleep duration in negative control group that received normal saline before pentobarbital was 23 ± 1.27 minutes (Figure 1). As expected, the reference drug diazepam was able to increase duration of sleep (48 ± 1 minutes, P < 0.001 vs. control). The HAE at doses of 50, 100, 150 and 200 mg/kg could significantly increase sleep duration to 43 ± 4.8 minutes (P < 0. 01), 60.83 ± 3.52 minutes (P < 0.001), 63.5 ± 5.42 minutes (P < 0.001) and 63 ± 3.4 minutes (P < 0.001), respectively. As expected, pretreatment of mice with flumazenil decreased the sleep-prolonging effect of diazepam (48 ± 1 minutes and 25.7 ± 1.2 minutes for diazepam and flumazenil + diazepam, respectively, P < 0.001). Similarly, the effect of HAE on sleep duration was significantly inhibited by flumazenil (63 ± 3.4 minutes and 19 ± 0.9 minutes for HAE and HAE + flumazenil, respectively, P < 0.001) (Figure 1).
DZP, diazepam; Ext, extract; Flu, flumazenil; Solvent, diazepam (3 mg/kg) and different doses (50, 100, 150 and 200 mg/kg) of the extract were intra-peritoneal administered 30 minutes before challenging animals with pentobarbital (30 mg/kg, i.p.). Flumazenil (2 mg/Kg) was used 15 minutes before the extract or diazepam. Data are mean ± SEM of 6 - 8 animals in each group. P < 0.01 (**); P < 0.001 (***) significantly different from control; P < 0.001 (###) significantly different from the same group plus flumazenil (2mg/Kg).
4.2. Effects of Nepeta glomerulosa on Sleep Latency
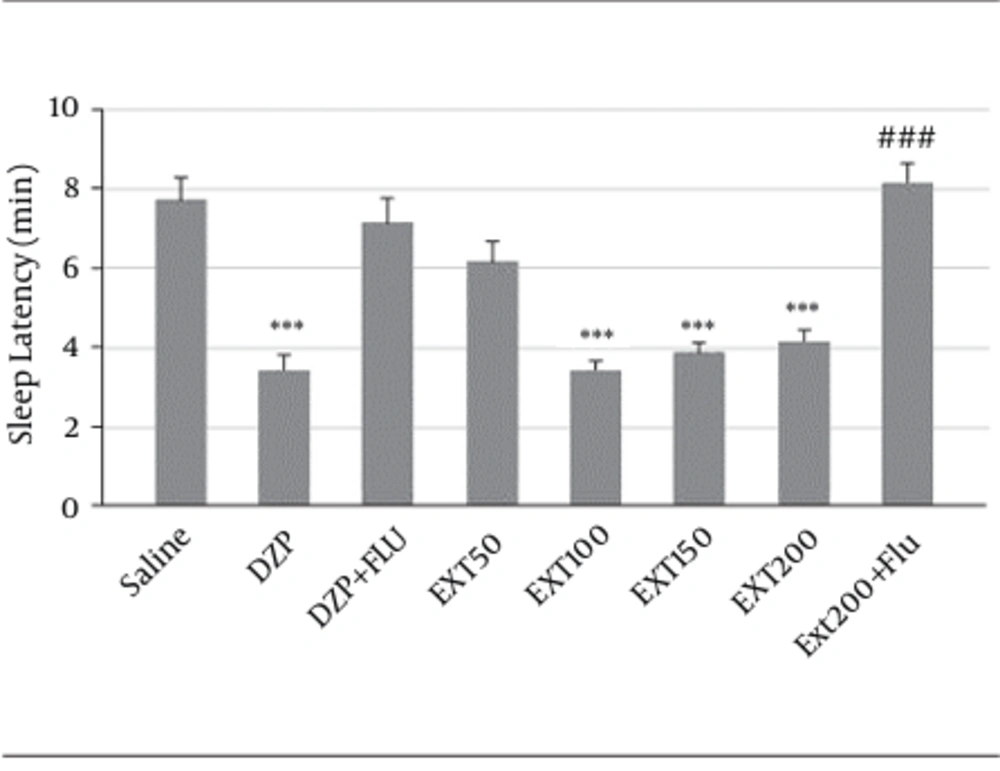
Diazepam (3.4 ± 0.36 minutes, P < 0.001) and HAE at doses of 100 (3.4 ± 0.2, P < 0.001) and 150 (3.85 ± 0.26, P < 0.001 mg/kg) and 200 (4.2 ± 0.26, P < 0.001) significantly reduced the latency to sleep in comparison to the saline (7.7 ± 0.56 minutes).
Figure 2 showed that flumazenil (2 mg/kg, i.p.) reversed the effects of diazepam (7.1 ± 0.63 minutes, vs. diazepam P < 0.001) and 200 mg/kg of HAE (8.14 ± 0.5 minutes, vs. HAE P < 0.001). Therefore, in flumazenil-treated mice, there was an increased latency to sleep in pentobarbital-induced hypnotic test.
DZP, diazepam; Ext, extract; Flu, flumazenil, Solvent, diazepam (3 mg/kg) and different doses (50, 100, 150 and 200 mg/kg) of the extract were intra-peritoneal administered 30 minutes before challenging animals with pentobarbital (30 mg/kg, i.p.). Flumazenil (2 mg/Kg) was used 15 minutes before the extract or diazepam. Data are mean ± SEM of 6 - 8 animals in each group. P < 0.01 (**); P < 0.001 (***) significantly different from control; P < 0.001 (###) significantly different from the same group plus flumazenil (2mg/Kg).
4.3. Effects of Fractions on Duration of Sleep
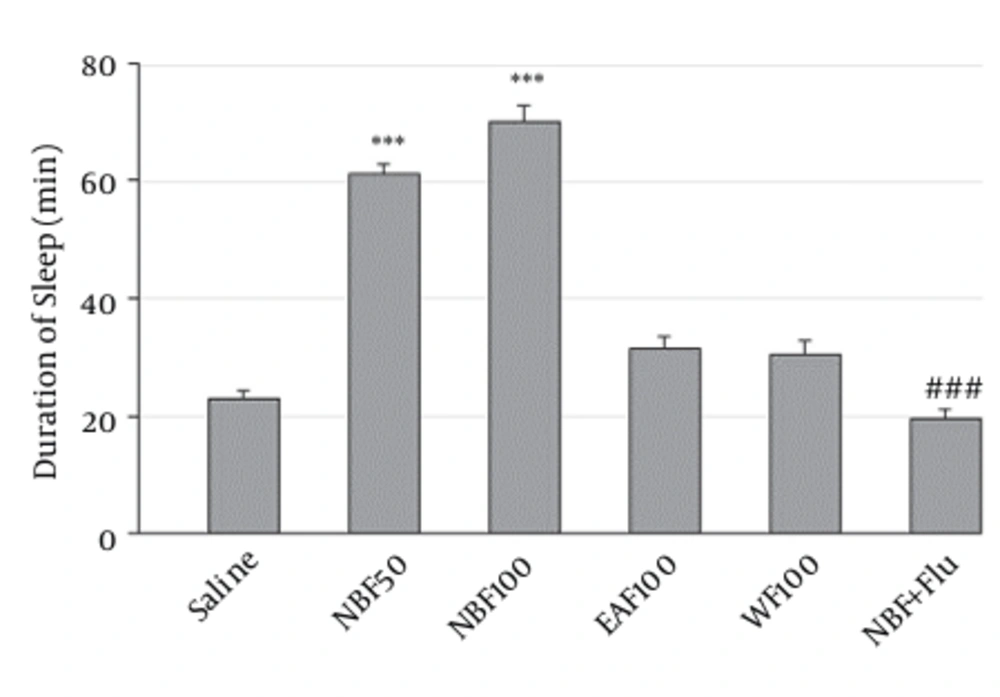
As shown in Figure 3, only NBF at doses of 50 mg/kg (61 ± 2 vs. control, P < 0.001) and 100 mg/kg (70 ± 2.6 vs. control, P < 0.001) was able to significantly increase sleep time. However, effects of WF and EAF were statistically non-significant. Also flumazenil reversed the effects of NBF (19.5 ± 1.6 minutes, vs. NBF P < 0.001).
The WF, EAF and NBF were intraperitoneally administered 30 minutes before challenging animals with pentobarbital (30 mg/kg, i.p.). Values are expressed as mean ± SEM. N = 6 - 8 animals in each group; P < 0.001 (***) significantly different from control; P< 0.001 (###) significantly different from the same group plus flumazenil (2 mg/Kg).
4.4. Effects of Fractions on Sleep Latency
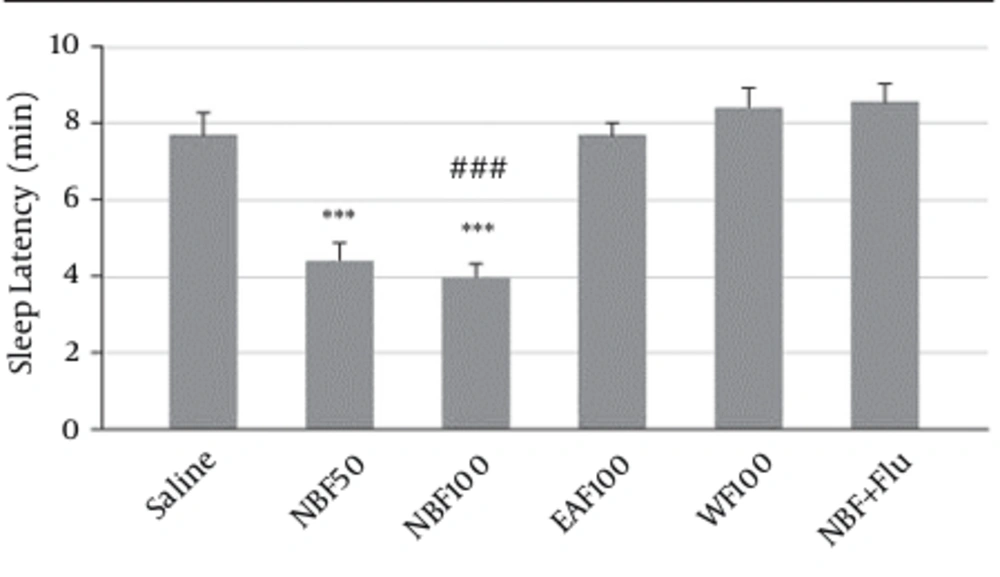
NBF at doses of 50 (4.4 ± 0.42, P < 0.001) and 100 (4 ± 0.3, P < 0.001) mg/kg significantly reduced the latency to sleep in comparison to the saline (7.7 ± 0.56 minutes). Also, in comparison with NBF group flumazenil reversed its effect on sleep latency (8.6 ± 0.5 minutes, vs. NBF P < 0.001) (Figure 4).
The WF, EAF and NBF were intraperitoneally administered 30 minutes before challenging animals with pentobarbital (30 mg/kg, i.p.).Values are expressed as mean ± SEM. N = 6 - 8 animals in each group; P < 0.001 (***) significantly different from control; P < 0.001 (###) significantly different from the same group plus flumazenil (2 mg/Kg).
4.5. Toxicity Assessments
The highest dose which did not kill any mice and the lowest dose which led to death of one mouse were 1.6 and 3.2 g/kg, respectively. The mean of these two doses (2.4 g/kg) was considered as LD50.
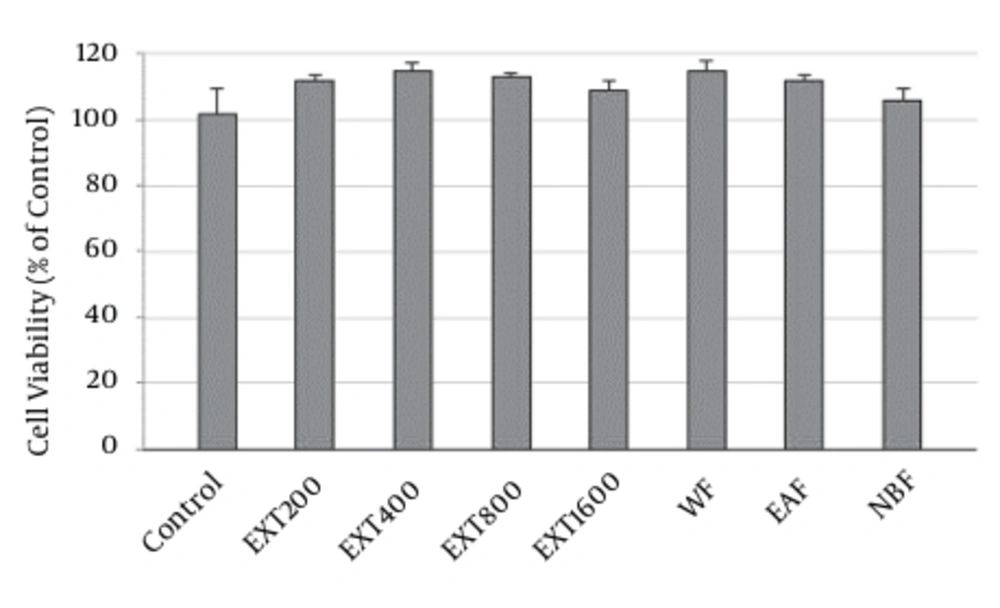
Result of MTT showed that none of the HAE concentrations decreased the proliferation of PC12 cells. In the presence of 200, 400, 800 and 1600 µg/mL of the extract, survival of the cells was 112 ± 1.5, 115 ± 2.1, 113 ± 1.3 and 109 ± 2.6%, respectively, as compared to control. Similarly, the HAE fractions exhibited no cytotoxicity. The level of viability was 115 ± 2.5%, 112 ± 1.6% and 106 ± 3.2% for WF, EAF and NBF (Figure 5).
5. Discussion
The genus Nepeta is one of the largest genera of the lamiaceae family. Several Nepeta are used in traditional medicine as antitussive, diuretic, diaphoretic, antispasmodic, febrifuge, anti-asthmatic and sedative agents (10-12). In this research we investigated the hypnotic effects of Nepeta glomerulosa for first time. Our results showed that hydro-alcoholic extract and n-butanol fraction increased the hypnotic effect of pentobarbital and decreased sleep latency. Also neurotoxicity test revealed, it did not have effect on cell viability. Diazepam belongs to the benzodiazepine group which has a binding site on GABA receptor type-ionophore complex (GABAA) (24) and this mechanism can be useful in the onset of sleep and increase of sleep duration (25). HAE administration at doses of 50 - 200 mg/kg produced sedative effect similar to that observed with 3 mg/kg of diazepam. However, it is possible for HAE to act via GABA ergic system. The inhibitory action of GABA is via opening of chloride channels and hyperpolarization of the membrane, all of them lead to CNS depression, sedative and hypnotic activity (26). Therefore, the drugs that influence these systems can be important in insomnia disorder.
In this research, the potentiated effect of HAE on sleep was observed. It not only prolonged the sleeping time, but also decreased the latency of falling asleep.
In order to determine if benzodiazepine receptor participates in the hypnotic effects of HAE, flumazenil as a specific antagonist of the benzodiazepine receptor was administered. Pre-treatment with flumazenil significantly reduced the effects of HAE. Therefore, it is possible for HAE to increase sleep via benzodiazepine receptor.
To obtain a better insight into the nature of the compounds responsible for the effect of HAE, three fractions were prepared: 1, The WF solubilizing the polar agents and water-soluble plant constituents (e.g. glycosides, quaternary alkaloids, tannins); 2, the EAF extracting compounds of intermediate polarity; and 3, the NBF bearing non-polar agents like sterols, alkanes and some terpenoids (19). The present data showed that NBF was the only fraction which could significantly prolong the sleep duration or decrease the sleep latency, also flumazenil reserved NBF effect on sleep duration. Therefore, it can be concluded that active compounds responsible for the effects of Nepetaglomerulosa are non-polar agents concentrated in NBF. A wide variety of phytochemicals has been reported to have sedative-hypnotic effects. These include terpenoids (e.g. linalool, eugenol), flavonoids (e.g. quercetrin, Luteolin), alkaloids (e.g. rosmarinic acid), steroids (e.g. beta-sitisterol) and saponins (27). Ingredients of Nepeta glomerulosa extract are alkaloid, saponin and oils (13). It’s oil have more than 35 constituents. The main constituents are as below: camphene (3.1%), α-humulene (3.2%), trans-α-bergamotene (3.5%), humulene epoxide (4.2%), trans-β-ocimene (4.7%), linalool (4.8%), caryophyllene oxide (8.0%), gernyl acetate (9.3%), limonene (9.7%),1,8-cineole (13.9%) and α-pinene (18.3%). (14, 15). Linck and coworkers demonstrated that linalool increases barbiturate-induced sleeping time in mice (28). Also, the studies have shown that some saponins of Nepeta glomerulosa have depression effects on CNS (13). Also, many Nepeta species have nepetalactones compounds that lead to CNS depression via GABA mediation (29). Isolated ursolic acid from some species of Nepeta increased pentobarbital effect and had depression effect on CNS (30). The toxicity assay showed that LD50 value for HAE of Nepeta glomerulosa is 2.4 g/kg. This dose is so far from its hypnotic doses (50 - 200 mg/kg). Also, HAE and fractions even at high concentrations did not decrease viability of neuronal. Therefore, it seems that hypnotic effect of Nepeta glomerulosa accompanied with no neurotoxicity.
In conclusion, this work showed that Nepeta glomerulosa had significant sedative-hypnotic effect. Further chemical and pharmacological analyses of the extract are needed to isolate and characterize the active components responsible for the sedative effect of Nepeta glomerulosa.