1. Background
Scrophularia is one of the largest genus of the Scrophulariaceae family commonly known as figworts, which is distributed widely in North America, central Europe, and Asia, especially the Mediterranean region. This genus includes about 200 species of flowering plants (1, 2).
Pharmacological activities such as wound healing, anti-inflammatory, antibacterial, diuretic, cardiovascular, anti-protozoa, fungicidal, analgesic, and cytotoxic have been reported in Scrophularia genus. The main secondary metabolites in Scrophularia genus have been identified as iridoids, phenylpropanoids, phenolic acids, and flavonoids (3-9).
Scrophularia striata belongs to the genus Scrophularia (Figure 1). It is an Iranian species and its local name is Teshne Dari (10). Traditionally, this herb has been used as an antibacterial agent to treat inflammation of the gastrointestinal tract, eye and ear infections, skin burns, hemorrhoids, Candida, inflammatory diseases, colds, respiratory, and inflammation of the gums and mouth. In the literature S. striata showed as anticancer, antibacterial, antifungal, and antidepressant properties (11-14). Three flavonoids (isorhamnetin-3-O-rutinoside, quercetin, and nepitrin), cinnamic acid, and one phenylpropanoid glycoside were isolated from the extract of S. striata (15).
The essential oil composition of S. striata collected from Lorestan (Kouhdasht) has been evaluated by Amiri et al. in 2011 and in this study we investigate the S. striata essential oil composition collected from Ilam. The comparison of the results with a previous study can complete the information of S. striata chemical composition (10).
Free radicals or reactive oxygen species (ROS) can damage the body as well as create diseases like cardiovascular, cancer, diabetes, and cirrhosis. The body can be removed free radicals generated in by its own natural antioxidant defense systems that include glutathione peroxidase, catalase, superoxide dismutase and etc., that are not completely efficient; therefore, dietary and natural antioxidants are required to reduce the effect of oxidative stress (16-18).
The phytochemical study and biological activities of the S. striata from Ilam have not been investigated. Therefore, the present study aimed to investigate the chemical composition, antioxidant properties, total flavonoid, and total phenolic content of the S. striata from Ilam.
2. Methods
2.1. Plant Material
The aerial parts of S. striata were collected from a wild population by randomized collection (Ilam, Iran), at an altitude of 1427 m. A voucher specimen (no. TUH-42801) is deposited in the central herbarium of University of Tehran, Tehran, Iran.
2.2. Extraction of the Essential Oil
The volatile oil of air-dried samples (80 g) was isolated by hydro distillation for 3 hours, using a Clevenger-type apparatus as previously reported (19). The volatile oil was dried by anhydrous sodium sulfate and, after filtration, stored at 5°C until analyzed.
2.3. GC and GC-MS Analyses
GC analysis was performed by using a ThermoQuest gas chromatograph with a flame ionization detector (FID). The temperatures of injector and detector were 250°C and 300°C, respectively. The analysis was carried out using fused silica capillary DB-5 column (60 m × 0.25 mm; film thickness 0.25 μm). The oven temperature was programed from 55°C to 260°C at the rate of 5°C/min, and finally held isothermally for 10 minutes. Nitrogen was used as a carrier gas at a flow rate of 1 mL min-1. GC-MS analysis was performed with the above-mentioned column by using ThermoQuest-Finnigan gas chromatograph coupled to a TRACE mass spectrometer, used under the same conditions. Ionization voltage was kept at 70 ev. Helium was used as the carrier gas. Interface and ion source temperatures were kept 250°C and 200°C, respectively. Mass range was scanned from 43 to 456 m/z (20).
2.4. Identification of Compounds
Composition of the essential oil was identified by calculation of their retention indices in a DB-5 column under the same chromatographic conditions for n-alkanes (C6-C24). Compounds were identified by comparing their mass spectra with authentic compounds or with those of the library. As well as for confirmed of identified compounds, their GC retention indices were compared with those of reported in the literature or with authentic compounds (21, 22).
2.5. Preparation of Extract
Air-dried samples (100 g) were extracted by methanol/water (50/50) using maceration method. The solvent of extract was removed and concentrated by rotary evaporator apparatus to produce a dark gummy solid (8 g). The extract was kept in a vial in a cool and dark place for other stages of the test.
2.6. Measurement of Free Radical Scavenging Activity (DPPH Assay)
Antioxidant activity was studied by measuring the scavenging activity with the solution DPPH, using the method of Naseri et al. (23). To prepare test solutions, one milliliter of DPPH solution (500 µM) was mixed with an equal volume of a solution of extract. The absorbance of the positive control (BHT) and blank (without sample) was read at 517 nm and room temperature on a Shimadzu UV-2100 spectrophotometer after 1 hour incubation without light. Each sample assay was a conclusion in triplicate and data is presented as a mean of the three values. Increased in DPPH radical scavenging activity showed with decrease in the absorbance of the DPPH solution. The values were calculated as a percentage using the following formula (24).
% DPPH radical scavenging = (absorbance of blank-absorbance of sample)/(absorbance of blank) × 100
2.7. Determination of Total Phenolic Content
The total phenolic content of the extract was measured by the Folin-Ciocalteu method (25). A total of 0.2 mL of extract (10 g/L) were mixed to 20 mL distilled water and 1 mL of Folin-Ciocalteu reagent after 3 minutes, followed by the addition of 3 mL of 7% (w/v) sodium carbonate. After 60 minutes, absorbance was measured at 765 nm. The total phenolic content was calculated from the gallic acid curve, and then, the content of phenolic compounds in extracts was expressed as mg of gallic acid equivalent per g dry weight.
2.8. Determination of Total Flavonoid Content
The total flavonoid content of extract was determined by the aluminum chloride colorimetric method (26). A total of 0.2 mL of extract (10 g/L) were mixed to 1.5 mL ethanol, 2.8 mL distilled water, 0.1 mL sodium acetate solution (1 M), and 0.1 mL of AlCl3 solution (10%). After 60 minutes, absorbance was measured at 415 nm. The total flavonoid content was calculated from quercetin curve, and the result was expressed as mg quercetin equivalent per g dry weight.
3. Results and Discussion
3.1. Essential Oil Composition
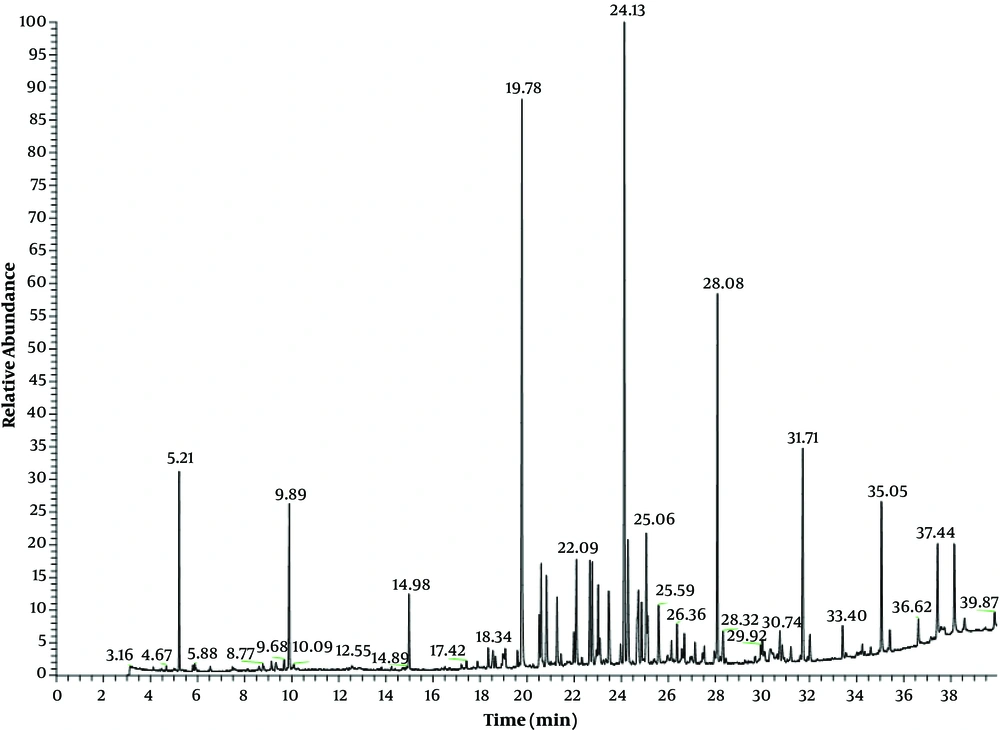
Hydrodistillation of dried aerial parts of S. striata afforded a light yellow color volatile oil in 0.2% yield (w/w %) relative to the dry weight of the plant. The identified compounds with quantitative result and the retention indices are listed in Table 1, where all compounds are organized in order of their elution on the DB-5 column. In total, 26 compounds were identified representing 93.1% of the total essential oil (Figure 2). The main identified compounds of the essential oil were n-hexane (16.3%), caryophyllene oxide (15.3%), spathulenol (13.1%), α-cadinol (12.3%), and docosane (6.3%) (Figure 3). The classification of compounds, based on their functional groups, is summarized in Table 1. High content of S. striata is identified by oxygenated sesquiterpene (48.9%).
| Compounds | RIa | Ilam, % | Lorestan, % |
|---|---|---|---|
| N-hexane | 623 | 16.3 | |
| Benzyl aldehyde | 952 | 0.7 | |
| l-octen-3-one | 972 | 0.4 | |
| 1-octen-3-ol | 974 | 1.9 | |
| Octanal | 998 | 0.7 | |
| N-decane | 1000 | 0.3 | |
| Limoneneb | 1024 | 0.6 | |
| Linalool | 1095 | 18.3 | |
| N-nonanal | 1098 | 0.4 | 3.1 |
| Trans-verbenol | 1140 | 0.5 | |
| α-terpineol | 1186 | 4.9 | |
| N-decanal | 1204 | 0.4 | 1.3 |
| Nerol | 1227 | 0.9 | |
| Carvone | 1242 | 1.1 | |
| 2-E-decanal | 1262 | 1.2 | |
| Geraniol | 1264 | 3.2 | |
| Trans-anethole | 1283 | 0.8 | |
| Carvacrolb | 1298 | 1 | |
| Tridecane | 1299 | 0.8 | |
| 2-methoxy-4-venyl phenol | 1313 | 1.1 | |
| 2E,4E-decadienel | 1315 | 0.4 | |
| Undecanol | 1367 | 1.2 | |
| α-copaene | 1374 | 0.6 | |
| β(E)-damascenone | 1386 | 5.9 | |
| Tetradecane | 1400 | 2 | |
| β-caryophyllene | 1418 | 3.7 | |
| Geranyl acetone | 1453 | 1.1 | |
| Germacrene D | 1484 | 4.7 | |
| E-β-Ionone | 1485 | 0.9 | |
| Methyl p, tert-butyl phenyl acetate | 1497 | 2.1 | |
| Bicyclogermacrene | 1500 | 1.1 | |
| δ-cadinene | 1522 | 0.7 | |
| Nerolidol | 1539 | 3.1 | |
| Dodecanoic acid | 1574 | 3.3 | |
| Spathulenol | 1576 | 13.1 | |
| Caryophyllene oxide | 1581 | 15.36 | 1.1 |
| Viridiflorol | 1590 | 2.3 | |
| Hexadecane | 1600 | 4.6 | |
| Humulene epoxide | 1608 | 1.8 | |
| α-Cadinol | 1653 | 12.3 | |
| Thujopsan-2-alpha-ol | 1660 | 1.1 | |
| Cadalene | 1674 | 0.3 | |
| 14-hydroxy-9epi-E-caryophyllene | 1676 | 3.1 | |
| Farnesol | 1712 | 0.4 | |
| Benzyl benzoate | 1762 | 1.6 | |
| Octadecane | 1800 | 2.3 | |
| Farnesyl acetate | 1818 | 0.6 | |
| 6-10-14-trimethyl pentadecan-2-one | 1847 | 8.4 | |
| 1-2-benzene dicarboxylic acid | 1872 | 3.9 | |
| Dibutyl phthalate | 1922 | 6.9 | |
| Farnesyl acetone | 1923 | 1.2 | |
| Phytol | 1936 | 2.1 | 1.5 |
| Ethyl hexadecanoate | 1983 | 0.3 | |
| Eicosane | 2000 | 0.7 | |
| Docosane | 2200 | 6.3 | |
| Nonacosane | 2900 | 0.4 | |
| Oxygenated sesquiterpene | 48.9 | 14.2 | |
| N-alkane | 33.5 | 0.4 | |
| Hydrocarbon sesquiterpene | 3.4 | 10.8 | |
| Oxygenated monoterpene | 3 | 35.7 | |
| Oxygenated diterpene | 2.1 | 1.5 | |
| Hydrocarbon monoterpene | 0.6 | ||
| Others | 1.2 | 27.7 |
aRetention indices relative to C6-C24 n-alkanes on the DB-5 column.
bThe identification was also confirmed by co-injection with an authentic samples.
Caryophyllene oxide, an oxygenated sesquiterpene, is commonly known as a preservative and antifungal against dermatophytes (27). Spathulenol is used as pesticides, antibacterial, and anti-fungal agent (28). α-Cadinol was identified as an anti-fungal, hepatoprotective agent and a possible remedy for drug resistant tuberculosis (29, 30).
The chemical composition of S. striata analysed in the present work was different to this species collecting from Lorestan. The main composition essential oil of S. striata from Lorestan contains linalool (18.3%), trimethylpenthadecane2-one (8.4%), di-n-butyl phthalate (6.9%), and beta damascene (5.9%) (10). This indicates that these compounds are different based on their collection location, which can result in factors such as environmental conditions and plant genotypes.
The chemical composition of S. striata essential oil analysed in the present work were compared to other species of Scrophularia genus. The main composition in S. ningpoensis were palmitic acid (25.40%), linoleic acid (10.04%), and α-linolenic acid (6.06%) as the most dominant fatty acids, while other main fatty acids were representatives of γ-linolenic acid (4.82%), cis-palmitoleic acid (3.94%), trans-oleic acid (3.52%), and cis-oleic acid (2.67%) (31).
The main composition in S. subaphylla were oxygenated monoterpenes, such as linalool (22.35%), phytol (15.74%), and geraniol (7.2%), as well as palmitic acid as a fatty acid (17.29%) (32).
The essential oil of S. oxysepala was characterized by a high content of aromatic compounds and phytol. The main constituents were phytol (25.3%), methyl benzyl alcohol (9.3%), dihydroeugenol (6.7%), methyl benzaldehyde (5.3%) and eugenol (1.3%) (33).
In S. amplexicaulis the essential oil composition was characterized by a high content of phenolic derivatives and oxygenated monoterpenes, such as eugenol (53.8%), eugenol acetate (24.5%), β-caryophyllene (5.7%), caryophyllene oxide (6.4%) and aromadendrene oxide II (2.1%) (34).
The main compositions of the autumn essential oil of S. frigida were palmitic acid (30.49%), phytol (12.99%), L-linalool (11.41%), and hexahydrofarnesyl acetone (6.65%). The summer essential oil of S. frigida was characterized by a high content of oxygenated monoterpenes, such as L-linalool (38.69%), geraniol (11.20%) and α-terpineol (9.99%), as well as palmitic acid (7.32%), as a fatty acid (35). This diversity in Scrophularia species essential oil composition can be used for further studies on the taxonomy of Scrophularia genus.
3.2. Antioxidant Activity, Total Phenolic and Total Flavonoid Content
The effect of antioxidant activity on DPPH radical scavenging is thought to be due to their radical scavenging activity or hydrogen donating ability. When a substance that can donate a hydrogen atom is mixed with solution of DPPH, this then gives rise to the reduced form diphenylhydrazine (no radical), with the loss of its violet color (36). A lower IC50 value indicates higher antioxidant activity. The DPPH scavenging abilities of the extract (IC50 = 85 µg mL-1) was lower than that of the synthetic antioxidant tert-butylated hydroxytoluene (IC50 BHT =26 µg mL-1). It is widely accepted that the antioxidant activity of a plant extract is correlated to its phenolic content (37). The total phenolic content of S. striata extract, calculated from the calibration curve (R2 = 0.9998), was 15.9 mg gallic acid equivalents/g dry weight, and the total flavonoid content (R2 = 0.9999) was expressed as 1.8 mg quercetin/g dry weight.
Antioxidant activity, total phenolic content, and total flavonoid content of S. striata suggests that the essential oil and hydro-alcoholic extract of S. striata has great potential for application as a natural antioxidant agent in pharmaceutical and food industry.