1. Background
Ischemic brain injury is one of the most important causes of disability and mortality in adults worldwide that results from cerebral circulatory arrest (1, 2). Transient global cerebral ischemia (TGCI) can cause cognitive, behavioral, learning and memory impairments (2, 3). Characterized mechanisms of post-TGCI neuronal damage consist of diminished blood flow to the brain, mitochondrial damage, free radical development, destruction of membrane, injury to the blood vessels, prevention of protein synthesis, inflammation, brain edema formation and finally delayed neuronal death of the CA1 zone of the hippocampus (4).
Ischemia/reperfusion injury via lipid peroxidation leads to increased reactive oxygen species (ROS) and therefore causes mitochondrial dysfunction, interruption of the membrane, alterations in permeability and ultimately cell death (5).
Nitric oxide (NO) is another factor involved in injuries after cerebral ischemia/reperfusion, that is generated by various sources such as neurons and glia (6), and can cause increased levels of ROS, consumption of adenosine triphosphate (ATP) and neuronal death (7).
There have been numerous researches concentrating on several neuroprotective agents for preventing the secondary neuronal damage following ischemia/reperfusion. Despite all efforts, at present we do not have an approved pharmacological therapy for preventing neuronal injuries following cerebral ischemia/reperfusion.
Dimethyl fumarate (DMF) is the methyl ester of fumaric acid with confirmed indications in patients with chronic plaque psoriasis, a dermatological sickness with immune system dysfunction and relapsing-remitting multiple sclerosis (8).
2. Objectives
The aim of this novel study was to investigate the effects of DMF on memory impairment and NO levels following cerebral ischemia in rats.
3. Methods
3.1. Chemicals and Drugs
Chemicals were bought from Sigma-Aldrich Chemical Co. As per the safety sheet issued by Sigma-Aldrich, Oral LD50 in rat is 2240 mg/kg.
DMF was dissolved in distilled water and was used at a dosage of 15 mg/kg body weight based on previous researches (9), twice daily (every 12 hours) via oral gavage for a period of 7 days after ischemia.
Chemical compounds studied in this article: DMF (PubChem CID: 637568).
3.2. Animal
The male Wistar rats, 200 - 250 g, were obtained from the animal house of Shahid Beheshti University of Medical Sciences, Tehran, Iran, and maintained under steady conditions (12 h/12 h light/dark cycle), at a controlled temperature of 23 ± 2ºC. Standard pellet diet and tap water were available.
3.3. Study Design
Rats were randomly allocated to three groups (N = 5). Group I included Sham-operated animals undergoing surgery without arterial occlusions, group II (control ischemic) received 0.2 - 0.25 mL of distilled water, twice a day by oral gavage, group III included rats undergoing surgery and cerebral ischemia and then received DMF (15 mg/kg, twice daily, for 1 week) by oral gavage.
3.4. Surgical Procedure
Before surgery procedure, rats were anesthetized with intraperitoneal administration of xylazine (5 mg/kg) and ketamine hydrochloride (50 mg/kg) (10).
Transient global cerebral ischemia was performed by common carotid arteries occlusion (CCAO) for 20 min, using the microvascular clamps. Subsequently, both clamps were removed and both arteries inspected for immediate reperfusion (11).
3.5. Morris Water Maze
The water maze is a circular tank 120 cm in diameter and 60 cm in height. The tank was filled with water (23 ± 2ºC) to a depth of 40 cm. The maze was located in a room containing extra-maze cues (posters). The maze was divided geographically into four quadrants (northeast (NE), northwest (NW), southeast (SE), southwest (SW)) and starting positions (north (N), south (S), east (E), west (W)) that were equally spaced around the perimeter of the pool. A hidden circular platform (diameter: 12 cm) was installed in the center of the NW quadrant, 1.5 cm below the surface of the water. A video camera was mounted directly above the maze to record the rats’ swim paths. A computer tracking system was used to measure the escape latency, time spent, traveled distance, the number of crossings, average proximity and average swimming speeds in the target quadrant (12, 13).
3.6. Measurement of Nitrite in the Hippocampus
The hippocampus was quickly removed following decapitation and homogenized (5:1 v/w) in ice-cold 0.1 M phosphate buffer, pH 7.4. The nitrite concentration in the hippocampal tissue was utilized as a measure of NO generation. Which was determined by measuring nitrite accumulation in the medium by Griess reagent (1% sulfanilamide and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride in 5% H3PO4). Then, 100 µL of supernatant and 100 μL Griess reagent were mixed and incubated for 5 min. The absorption was estimated in a microplate reader at 540 nm (14).
3.7. Statistical Analysis
All the results are presented as mean ± SEM. Statistical analyses were carried out using PRISM 6.0. Analysis of variance test was applied to determine statistical significance. A P value < 0.05 was regarded as statistically significant.
4. Results
4.1. Effect of DMF on Spatial Memory in the Morris Water Maze Test
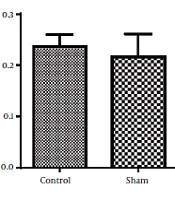
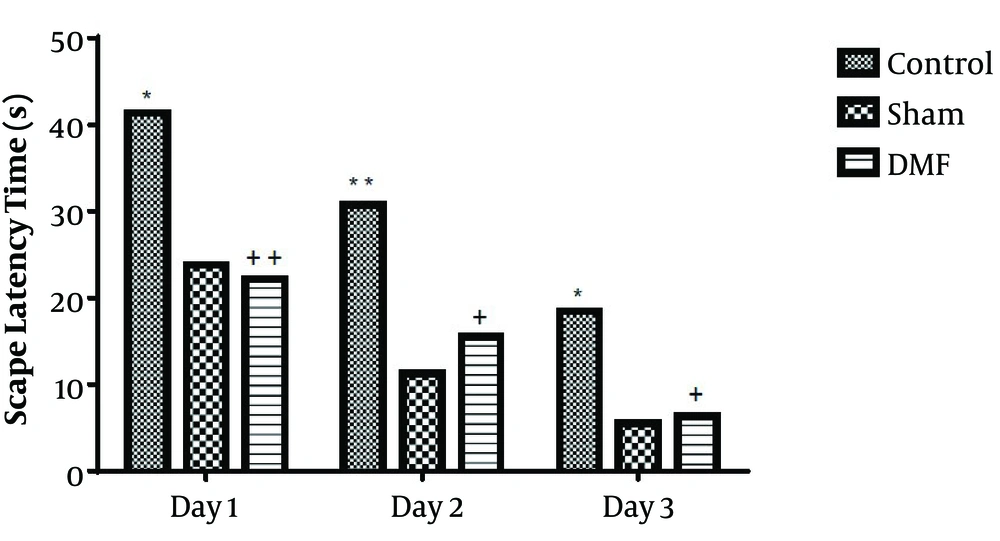
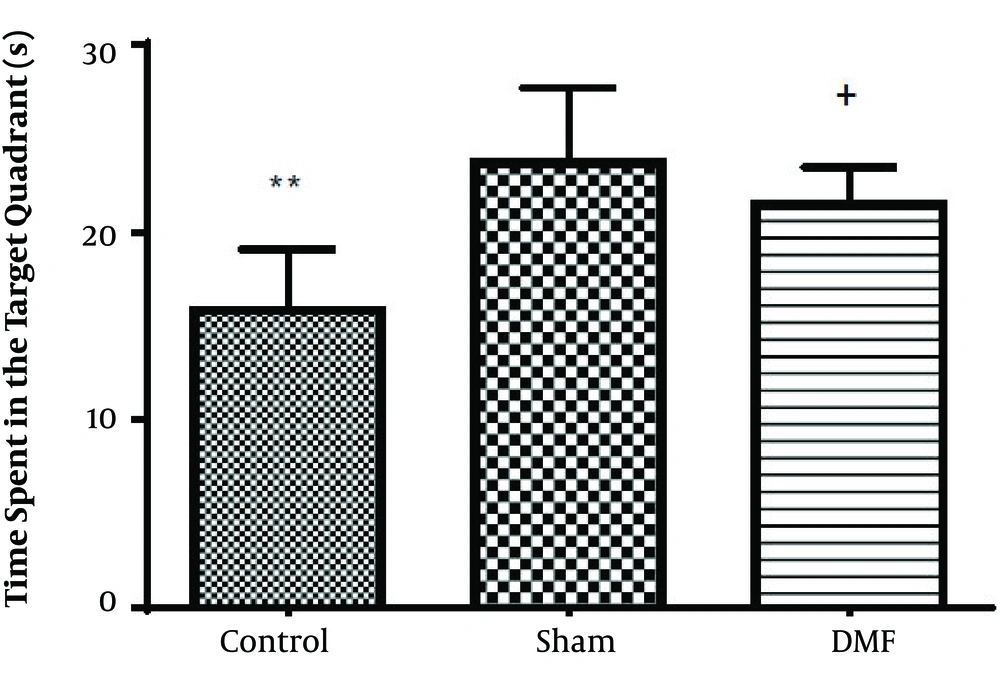
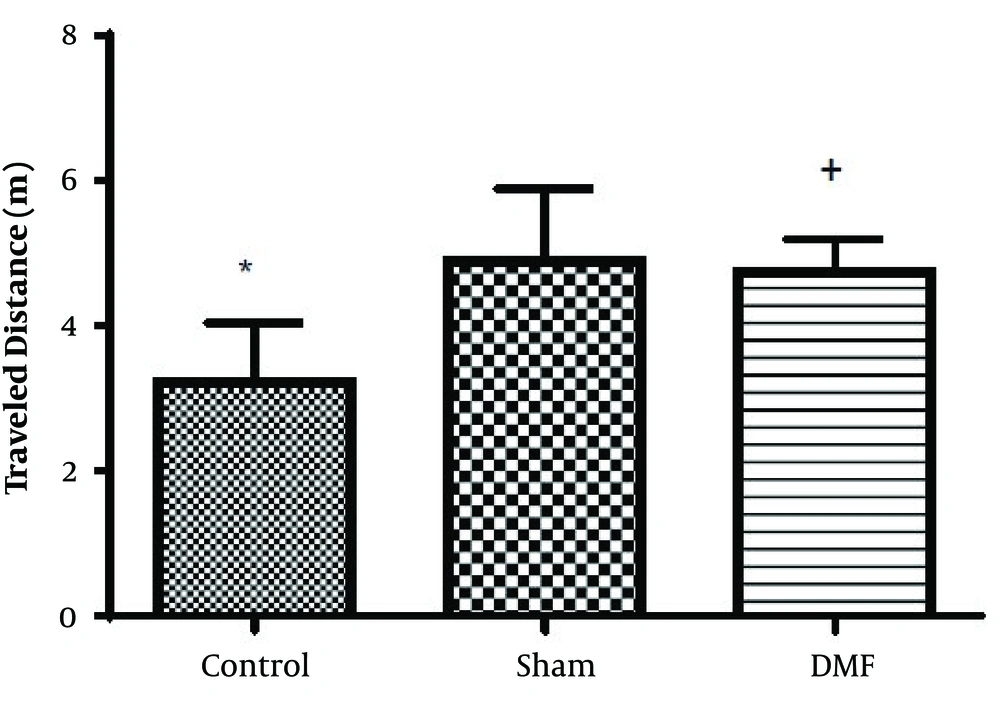
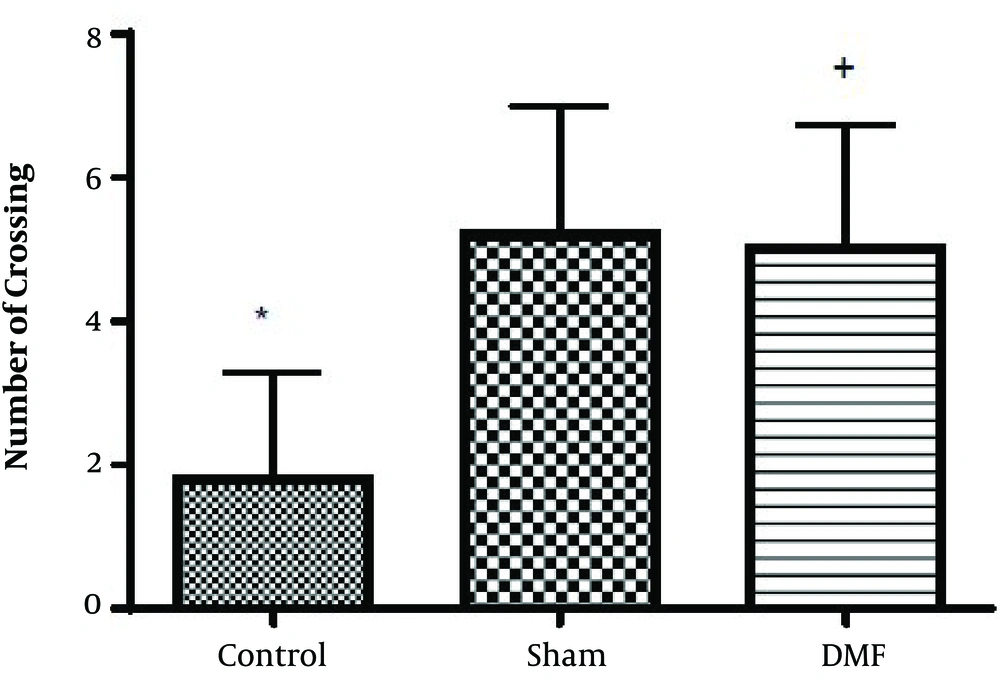
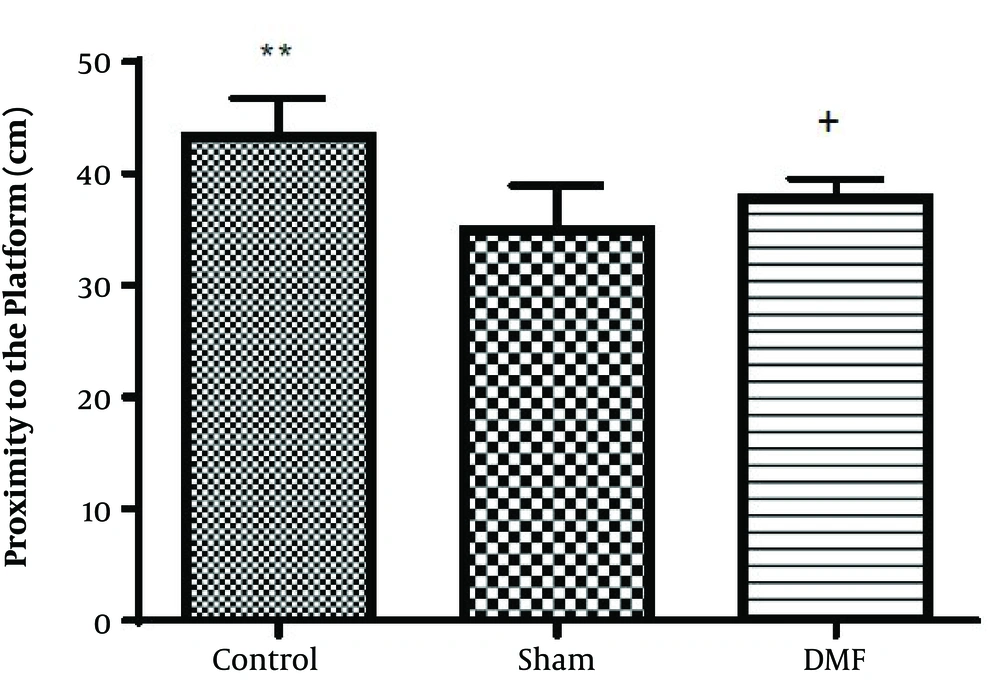
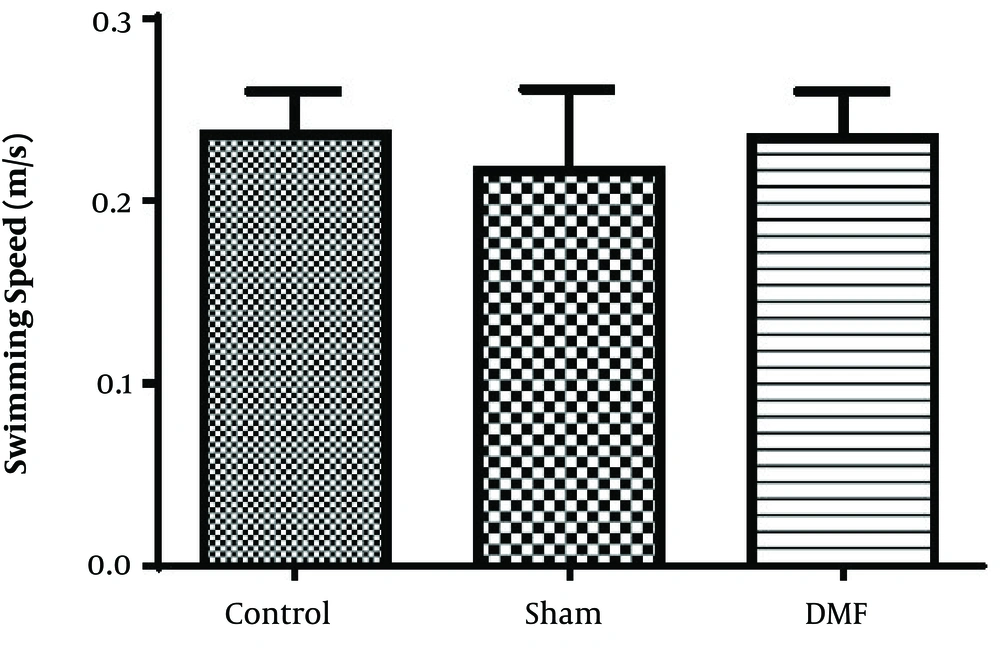
Results analysis of the Morris water maze during training trials (first 3 days), escape latency (time to find the hidden platform) in DMF (15 mg/kg) and sham groups were significantly decreased (P < 0.05) in comparison with control group. eEscape latency on the third day of the training trial was the lowest (Figure 1). Additionally in probe trial, time spent and traveled distance in goal quadrant during the experiment, in DMF and sham groups were significantly increased (P < 0.05) in comparison with control group (Figures 2 and 3, respectively) Furthermore, in the probe trial the number of crossings on the platform’s place was significantly enhanced (P < 0.05) in DMF and sham groups in comparison with control group (Figure 4). Moreover, in the probe trial, mean proximity to platform in DMF (15 mg/kg) was significantly different compared to the control group (P < 0.05) (Figure 5) but average swimming speed during the probe trial in Morris water maze test, did not show a significant difference between groups (Figure 6).
Escape latency time (mean ± SEM) during training days, to reach a hidden platform in rats treated by DMF (15 mg/kg, orally, twice daily, for 1 week) in the Morris water maze test. By Two-way ANOVA, *P < 0.05, **P < 0.01, significantly different compared to the sham, +P < 0.05, ++P < 0.01, compared with the control group.
4.2. Effect of DMF on NO Levels
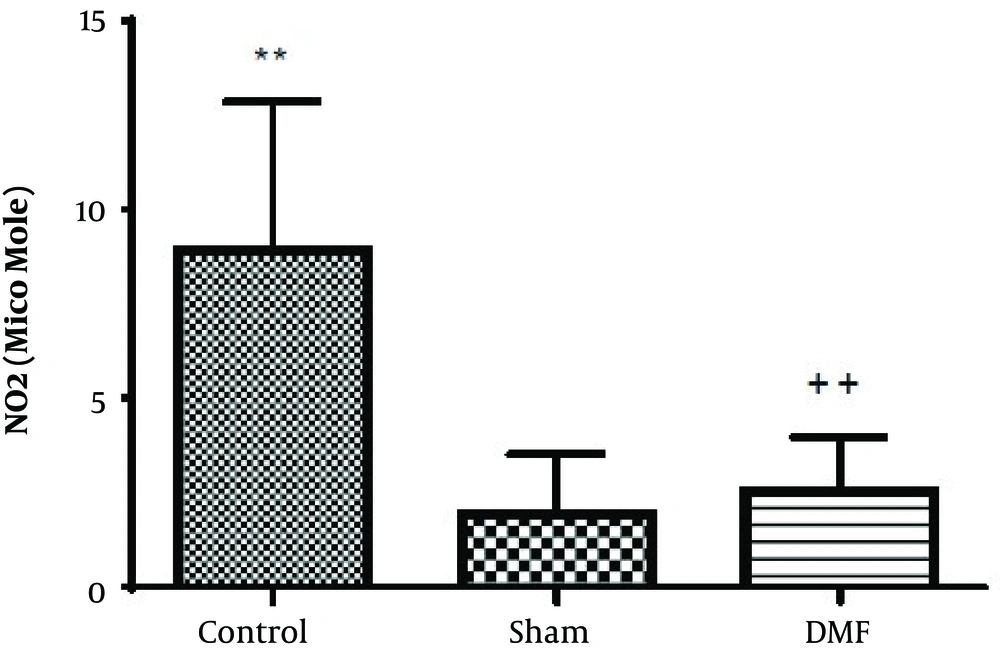
According to the one-way ANOVA results of this study, nitric oxide level of the hippocampus of control group was increased compared to the sham group. But DMF (15 mg/kg, twice daily, for 1 week) prevented the rise of the levels of NO [F (2, 12) = 11.18, P < 0.01] in the hippocampus of rats subjected to ischemia/reperfusion injury (Figure 7).
Effect of treatment with DMF (15 mg/kg, orally, twice daily, for 1 week) on the concentration of Nitric Oxide in the hippocampus of rats subjected to transient cerebral ischemia/reperfusion. Data are reported as mean ± SEM; (n = 5/group). **P < 0.01 compared with sham group; ++P < 0.01 compared with the control group.
5. Discussion
Our research proposed the use of DMF for the management and prevention of memory impairments caused by cerebral ischemia/reperfusion. In addition, we investigated the underlying mechanism of DMF with a focus on nitrosative stress. Learning and memory impairments represent the pathogenic advancement of the damage induced by cerebral ischemia/reperfusion. Such damages could lead to disabilities and restriction in social activities (3, 15). In our study, we have seen a progress in learning behaviors in rats treated with DMF compared to control group, 7 days after the induction of ischemia by Morris water maze test.
Previously many studies have focused to nominate a pharmacological intervention to attenuate cerebral ischemia injury and subsequent memory impairments and to address that goal, several compounds such as ROS scavengers, glutamate receptor blockers, calcium channel inhibitors were applied (16), but none of them lead to an approved medication for the discussed indication, although experimental studies on numerous neuroprotective and anti-inflammatory agents are still ongoing.
The results obtained from Morris water maze, showed that the rats treated with oral DMF, improved spatial memory, represented by reduction in escape latency (time to find the platform) and proximity of rats to the platform’s location as compared to the ischemia group. Also, DMF significantly enhanced time spent in the target quadrant, traveled distance and number of crossings in the goal quadrant in comparison with the ischemia group. No significant difference was found in swimming speed between the ischemia, DMF and Sham group in the probe trial, on the 4th day of Morris water maze test. Hence the concern that motor impairment upon surgery could affect our data, can be excluded. From the other side as a result, since the mean speeds of the rats in trial day were not differ significantly between groups, the effects of DMF on spatial navigation do not appear to be relevant to improvement in motor ability.
Also in experiment comparing of escape latency in each day, we observed that the escape latency on the third day in the DMT-treated rats was significantly shorter in comparison with the control group. The latter finding also supports the other data that DMF might be able to improve learning functionality of ischemic rats.
Enhanced levels of NO have been shown as another factor involved in the injuries associated with cerebral ischemia/reperfusion and may worsen the primary damages to neurons (17, 18). Furthermore, NO reacts with superoxide anion radical (O2−) to produce per-oxynitrite, a very reactive molecule in lipid oxidation, proteins and DNA; that again leads to further increased inflammation, microglial cells destruction and CNS injury (19, 20).
One of the major findings of our research, is that DMF can prevent increased levels of NO after cerebral ischemia/reperfusion in rats hence, prevention of the boost of NO, can be considered as another beneficial effect of DMF in complications resulting from cerebral ischemia. Some other studies have shown that prevention of increased NO levels by various compounds including DMF can be employed to restrain the progression of neurodegenerative diseases (21-23).
5.1. Conclusions
Our results demonstrate that DMF improves cerebral ischemia-induced learning and memory impairment, and the promising memory-improvement effects of DMF may be mediated through reduction of the levels of NO in the hippocampus.