1. Background
Conventional chemotherapy typically functions by inhibiting cell growth, leading to the death of dividing cancer cells and rapidly growing normal cells. Chemotherapy for cancer often damages fast-growing tissues, such as blood cells, leading to serious side effects (1). A common adverse effect associated with chemotherapeutic agents is chemotherapy-induced peripheral neuropathy (CIPN), characterized by sensory (hyperalgesia, allodynia, paresthesia, and spontaneous pain), motor (weakness, incoordination), and autonomic symptoms (2). The onset of CIPN diminishes the quality of life for patients undergoing treatment and can result in dose limitations, the discontinuation of chemotherapy, and the necessity for intensive clinical care (3). Vincristine is one of the most commonly used chemotherapeutic agents, especially for solid tumors, and is known to cause greater neurotoxicity than other vinca alkaloids (4). The use of vincristine-induced neuropathic pain in rats as a model often serves to study neuropathic pain and closely resembles the symptoms associated with cancer chemotherapy in humans (5). Neuroleptic drugs, analgesics, tricyclic anticonvulsants, antidepressants, and opioids have been reported to offer some benefits in managing neuropathic pain (6). However, an effective treatment for established CIPN has not yet been identified, although various prevention and treatment strategies are being explored (7). Herbal remedies have proven to be valuable for managing various types of neuropathic pain. Among these, Curcuma longa, Ocimum sanctum, Vernonia cinerea, and Lavandula angustifolia have demonstrated beneficial effects in treating neuropathic pain induced by vincristine (8-11).
Capparis spinosa L., commonly known as caper, is a perennial shrub native to the Mediterranean region, with its distribution extending from Morocco to Armenia and Iran (12). This plant has been documented to exhibit various biological activities, including anti-diabetic (13), anti-inflammatory (14), anti-hepatotoxic (15), and anti-hypertensive effects (16). In this study, caper was chosen for its analgesic and anti-inflammatory properties demonstrated in animal studies (17), as well as its traditional use in Persian medicine for treating neuropathic symptoms such as paresthesia, numbness, and tingling.
2. Objectives
The study aimed to evaluate its efficacy on peripheral neuropathy induced by vincristine. To achieve this, a hydroalcoholic extract of caper fruit was prepared, and various behavioral tests were conducted to assess muscle strength, hyperalgesia, and mechanical allodynia.
3. Methods
3.1. Reagents
Vincristine sulfate (Sobhan Oncology Company, Iran), pregabalin (Sobhan Drug Company, Iran), naloxone (Tolidaru Pharmaceutical Company, Iran), and cyproheptadine (Tehrandarou Pharmaceutical Company, Iran) were administered to animals either orally or intraperitoneally. The Capparis spinosa extract was administered orally via gavage. All test solutions were prepared immediately before the commencement of the tests.
3.2. Preparation of Extracts
The fruits of Capparis spinosa, purchased from the market, were verified by a botanist (Voucher No. HMS535) and stored in the herbarium of the Traditional Medicine and Materia Medica Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. To prepare the extract, the dried fruits were ground into a powder and soaked in 70% ethanol overnight with agitation; this process was repeated 3 times consecutively. The extract was then filtered, and the solvent removed using a rotary evaporator and freeze dryer, yielding an extraction rate of 21%. The final dried extract was stored at 4°C until needed (18).
3.3. Animals
Male Wistar rats weighing between 200 - 250 g were chosen for this study. The animals had ad libitum access to food and water and were acclimatized to a 12-hour light/dark cycle. The experimental protocols received approval from the Institutional Animal Ethics Committee, and the experiments and care of the animals were conducted in accordance with the ethical guidelines of the Animal Care and Use Committee (IACUC) of Shahid Beheshti University of Medical Sciences, under the approval code IR.SBMU.RETECH.REC.1396.469.
3.4. Experimental Design
Forty-eight male Wistar rats were randomly assigned into 8 groups. Initially, the animals underwent the open field test, followed by identical animals being subjected to the footprint, von Frey, hot plate, and grip strength tests. The first group, consisting of healthy untreated rats, served as the control group and received daily administrations of 0.1 mL/kg of normal saline (i.p.). The second group, designated as neuropathic rats, was administered 0.1 mg/kg/day of vincristine sulfate (i.p.) for 2 weeks (19, 20). Three additional test groups received daily doses of 0.1 mg/kg of vincristine sulfate (i.p.) along with 100, 200, and 400 mg/kg of caper extract (orally), respectively, for the same 2-week period. The final group was treated with 0.1 mg/kg of vincristine sulfate (i.p.) followed by 20 mg/kg of pregabalin (p.o.) for the same duration.
To investigate the potential mechanisms of action of the extract, naloxone, an opioid receptor antagonist (1 mg/kg), and cyproheptadine, a serotonin receptor antagonist (5 mg/kg), were administered intraperitoneally after administering an effective dose of the extract (400 mg/kg).
3.5. Open Field Test
The open field test was chosen to assess the general motor activity of the rats. For this purpose, after treatment, animals were placed at the center of a Plexiglas box measuring 60 × 60 × 60 cm and observed for 10 minutes. A digital camera mounted above the apparatus and connected to a computer recorded the rats' movements during this time. The testing area was thoroughly cleaned with 70% ethyl alcohol before conducting subsequent experiments. All recorded videos were analyzed using Ethovision XT software (Noldus, The Netherlands), and the total distance moved by the animals was calculated (21-23).
3.6. Footprint Test
To assess the recovery of locomotor activity, the footprint test was conducted 14 days after administering the extracts and vincristine. Following acclimatization to the environment, the hind limbs of the animals were dipped in ink. Subsequently, the animals walked on a white paper surface fitted within the apparatus, and their footprints were analyzed using Image J software (24).
3.7. Grip Strength Test
The muscle strength of the rats following vincristine injection was evaluated using the grip strength test. During this test, the rats grasped a metal wire with their front limbs while being gently pulled by their tails. The force exerted by the animals on the wire was measured and recorded. This procedure was repeated three times for each rat, with 5-minute intervals between repetitions (25).
3.8. Von Frey Hair Test
This stage involved using a set of von Frey filaments to assess the withdrawal threshold of the hind limbs. The rats were placed in a Plexiglas box (Sanat Azma Tower, Iran) for 30 minutes to acclimatize. Then, the soles of their feet were prodded with von Frey filaments (Stoelting, USA). The test was conducted three times, and the average calculated time was regarded as the response to the test (26).
3.9. Hot Plate Test
The hot plate method, as described previously by Eddy et al. (27), was utilized to measure thermal hyperalgesia. In this experiment, rats were placed on a hot plate maintained at 52.5 ± 0.5°C. The withdrawal latency of the hind paws in response to the heat, with a cut-off time of 40 seconds, was noted. The recorded time was considered the pain threshold. Delays in retracting the hind paw or jumping off the surface of the hot plate were documented.
3.10. Estimation of Total Phenolic Content
The total phenolic content in the caper fruit extract was determined using the Folin-Ciocalteu method. Gallic acid, prepared as a reference standard at various concentrations (25, 50, 75, 100, 150, and 200 µg) in 1 mL of ethanol and 400 µg of the hydroalcoholic extract of Capparis spinosa dissolved in 1 mL of ethanol, were used. One milligram of both the standard solution and the extract was mixed with 5 mL of Folin-Ciocalteu reagent diluted in distilled water (1:10). After 15 minutes, 4 mL of aqueous Na2CO3 (1 M) was added to the mixture. The solutions were then incubated at room temperature for 30 minutes. The absorbance of the mixtures was measured at 760 nm, and a calibration curve was plotted. The total phenolic content of the extract was calculated and expressed as milligrams of Gallic acid equivalent per gram of extract (mg GAE/g) (28).
3.11. Estimation of Total Flavonoids
The total flavonoid content of the caper fruit extract was estimated using aluminum chloride. For this analysis, a series of rutin concentrations (25, 50, 75, 100, and 150 µg) in 1 mL of methanol as a reference standard and 400 µg of the extract in 1 mL of methanol were prepared. Both the standards and the extract were mixed with 2.5 mL of AlCl3 (1:1 distilled water and methanol) and left to stand at room temperature for 40 minutes. Subsequently, a calibration curve was constructed based on the absorbance of rutin at 415 nm, and the total flavonoid content of the extract was expressed as milligrams of rutin equivalents per gram of sample (mg rutin/g) (29).
3.12. Statistical Analysis
Data were analyzed using GraphPad Prism (version 8) software. Results were presented as mean ± SEM and were subjected to one-way ANOVA followed by Tukey’s multiple comparison tests. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Effect of Capparis spinosa Extract on Open Field Test
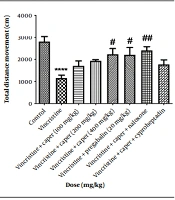
The motor activity of the rats was evaluated by measuring the total movement within the open field box. Compared to the control group, motor dysfunction induced by vincristine was evident, as illustrated in Figure 1. Furthermore, the caper extract at a dose of 400 mg/kg and pregabalin (20 mg/kg) were able to restore movement ability. In addition, naloxone and cyproheptadine were ineffective in reversing the extract's effect.
Capparis spinosa extracts effect (100, 200, 400 mg/kg) on vincristine-induced neuropathic pain evaluated by open field test. Data are represented as mean ± SEM, n = 8. Significant difference in comparison with the control group displays as **** P < 0.0001 and comparison with the vincristine group displays as ## P < 0.001 and # P < 0.05.
4.2. Effect of Capparis spinosa Extract on Footprint Test
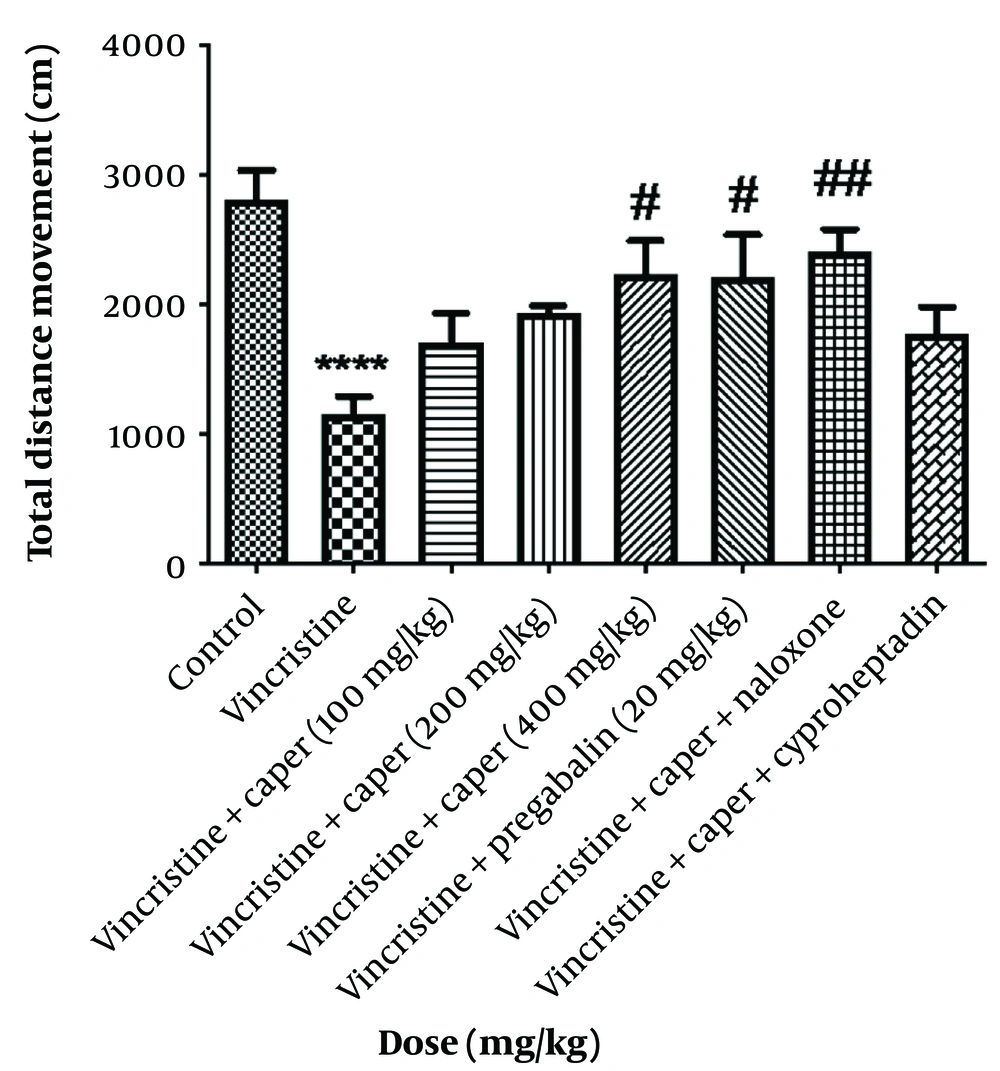
As shown in Figure 2, the foot pressure exerted on the surface, quantified by pixel values, decreased following vincristine administration (2264.6 ± 412.2 pixels). The pixel values representing the steps increased significantly after administration of the extract at a dose of 400 mg/kg and pregabalin (5374.7 ± 408.2 and 6711.6 ± 1000.3 pixels, respectively). Nonetheless, injections of naloxone and cyproheptadine were unable to modify this effect.
Capparis spinosa extracts effect (100, 200, 400 mg/kg) on vincristine-induced neuropathic pain evaluated by footprint test. Data are represented as mean ± SEM, n = 8. Significant differences in comparison with the control group displays as **** P < 0.0001 and comparison with the vincristine group displays as ### P < 0.001 and # P < 0.05.
4.3. Effect of C. spinosa Extract on Grip Strength
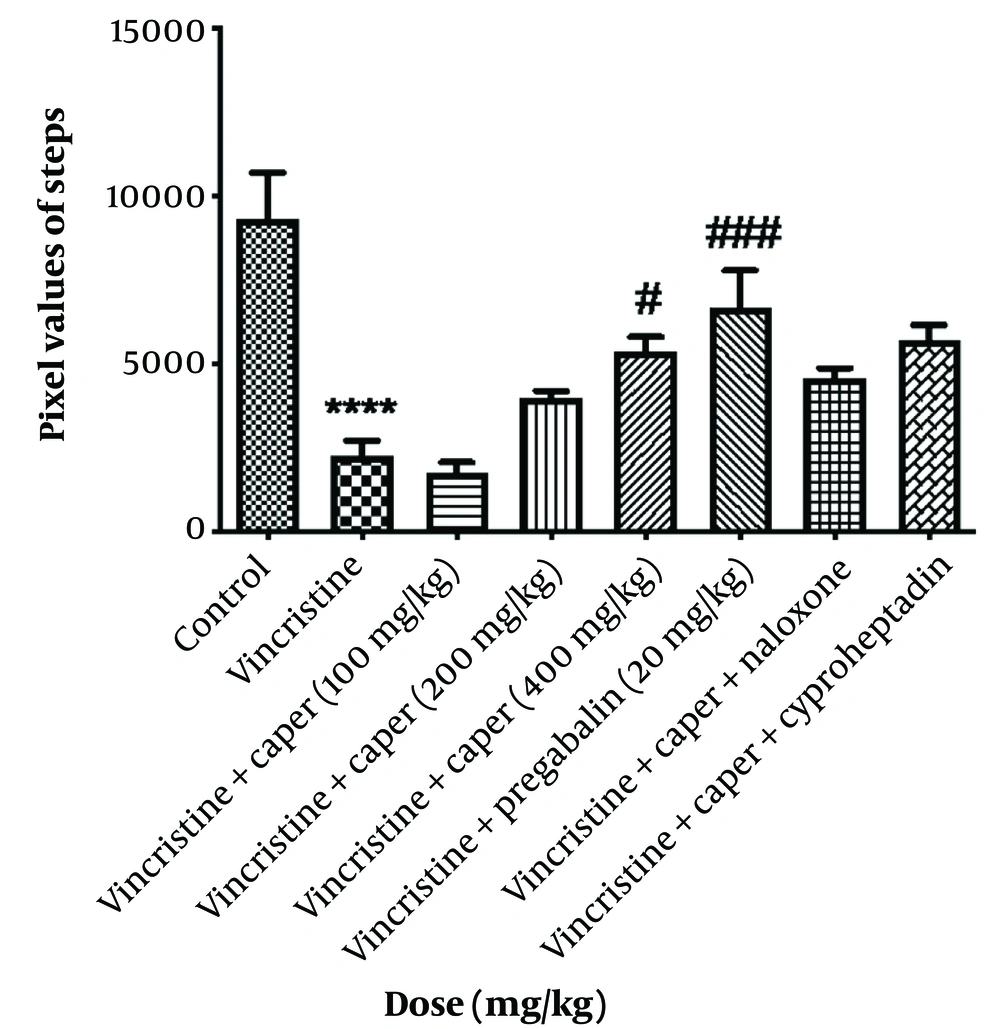
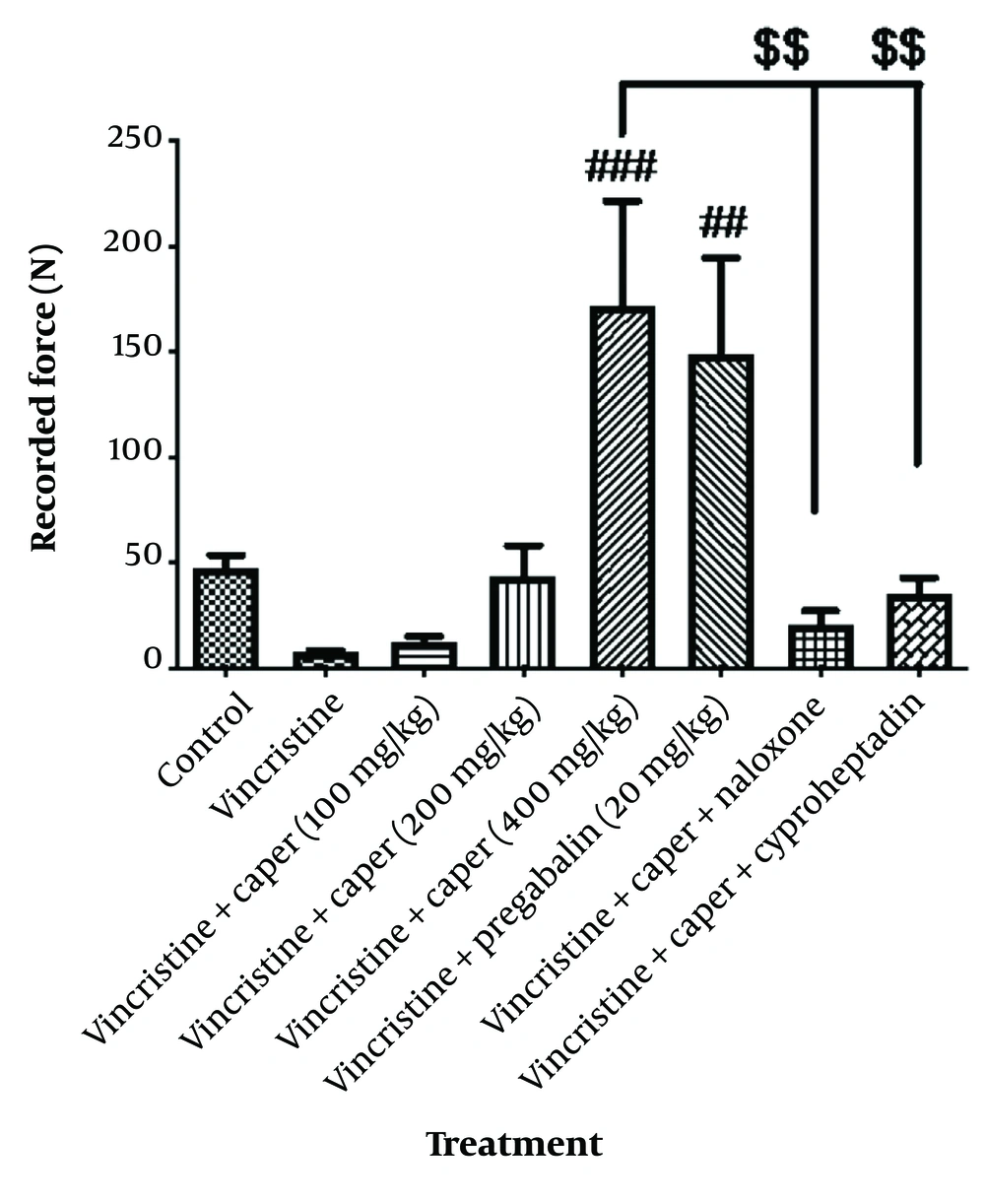
The muscle strength of the rats, after receiving vincristine, was evaluated using the grip strength test and showed a significant decrease compared to that of normal rats (Figure 3). Among the doses of the extract, 200 and 400 mg/kg were effective in recovering the muscle weakness induced by vincristine administration. Furthermore, pregabalin at a dose of 20 mg/kg failed to restore the lost muscle strength. Naloxone and cyproheptadine significantly reduced the muscle-strengthening effect of the extract.
Capparisspinosa extracts effect (100, 200, 400 mg/kg) on vincristine-induced neuropathic pain evaluated by grip strength test. Data are represented as mean ± SEM, n = 8. Significant difference in comparison with the control group displays as **** P < 0.0001 and comparison with the vincristine group displays as ##P < 0.001 and # P < 0.05. A significant difference in comparison between naloxone and cyproheptadine groups with the vincristine-treated groups displays as $$$ P < 0.001, $$$$ P < 0.0001.
4.4. Effect of Capparis spinosa Extract on von Frey Hair Test
The mechanical nociceptive threshold, assessed using von Frey filaments, exhibited a significant decrease in response to tactile allodynia in animals administered vincristine compared to the control group (Figure 4). Conversely, animals treated with 400 mg/kg of the extract showed a significant improvement in paw withdrawal response relative to the vincristine group. Similarly, the mechanical hypersensitivity induced by vincristine was reduced in the group treated with pregabalin. Both naloxone and cyproheptadine significantly reduced the enhanced threshold response to tactile stimulus brought about by the extracts.
Capparis spinosa extracts effect (100, 200, 400 mg/kg) on vincristine-induced neuropathic pain evaluated by von Frey test. Data are represented as mean ± SEM, n = 8. A significant difference in comparison with the vincristine group displayed as ### P < 0.001 and ## P < 0.01. A significant difference in comparison between naloxone and cyproheptadine groups with the vincristine-treated groups displayed as $$ P < 0.01.
4.5. Effect of Capparis spinosa Extract on Hot Plate Test
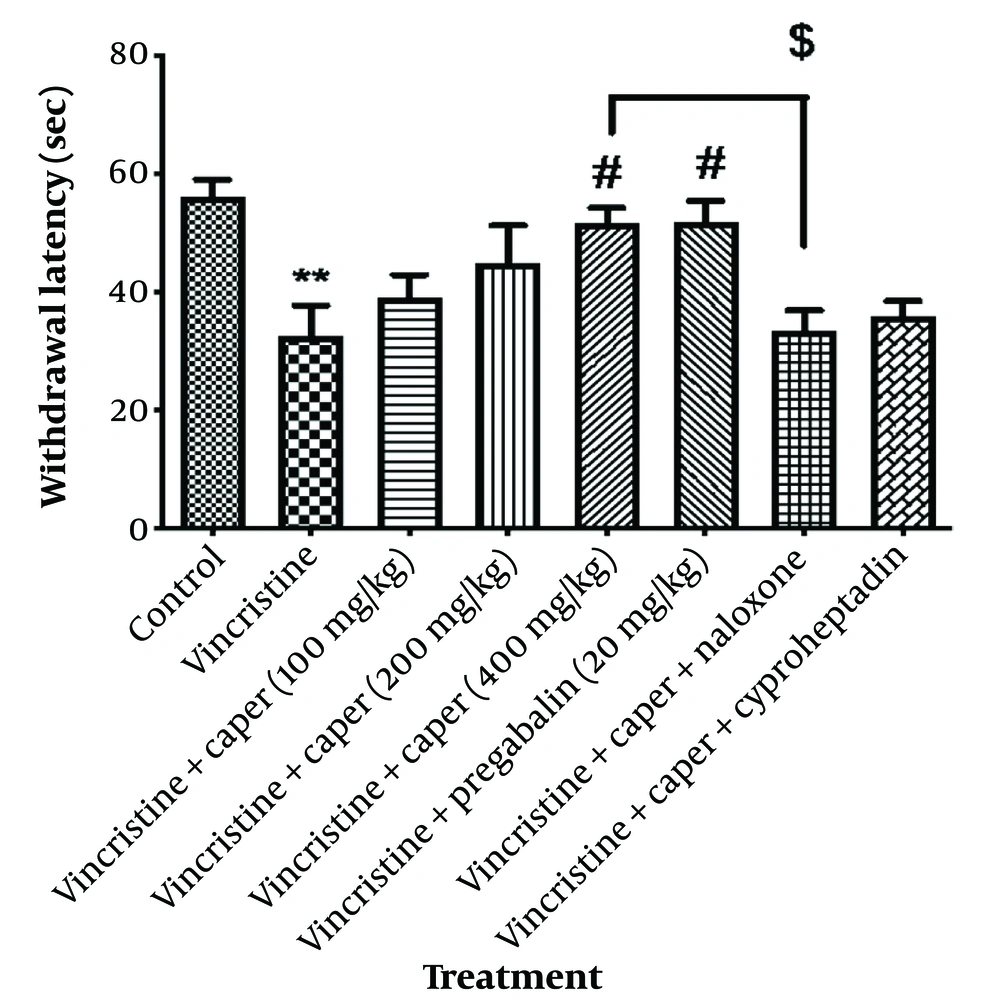
A significant reduction of reaction time to heat stimulus in the group that received vincristine compared to the control group indicated the effect of vincristine in decreasing response time to heat (hyperalgesia). As shown in Figure 5, oral administration of the extract at the dose of 400 mg/kg remarkably prolonged the reaction time to heat after administration of vincristine and this effect (51.8 ± 2.4 s) was similar to the effect of pregabalin 20 mg/kg (51.9 ± 3.6 s). Naloxone decreased the reaction time to heat stimuli in rats treated with a 400 mg/kg dose of the extract.
Capparis spinosa extracts' effect (100, 200, 400 mg/kg) on vincristine-induced neuropathic pain was evaluated by a hot plate test. Data are expressed as mean ± SEM, n = 8. A significant difference in comparison with the control group displayed as ** P < 0.01 and in comparison with the vincristine group displayed as # P < 0.05. The significant difference in comparison between the naloxone group and the vincristine-treated groups was displayed as $ P < 0.05.
4.6. Determination of Total Phenol Content
The hydroalcoholic extract of Capparis spinosa was determined to have a total phenolic content of 169.402 ± 8.979 µg of gallic acid equivalent per gram of the dried extract.
4.7. Determination of Total Flavonoid Content
The findings showed that the hydroalcoholic extract of Capparisspinosa contains a total flavonoid content of 73.662 ± 2.321 µg of rutin equivalent per gram of the dried extract.
5. Discussion
The current study aimed to evaluate the effect of the hydroalcoholic extract of Capparis spinosa fruits on chemotherapy-induced neuropathy. The extract significantly reduced vincristine-induced neuralgia and motor neuropathy across various tests, including heat hyperalgesia and mechanical allodynia, such as the hot plate, open field, von Frey, grip strength, and footprint tests. In this study, pregabalin, a well-known antiepileptic drug recognized for its efficacy in alleviating allodynia, hyperalgesia, and painful peripheral neuropathic conditions, was used as a benchmark to compare the effectiveness of the extract in treating CIPN (30, 31). The administration of the hydroalcoholic extract of caper at a dose of 400 mg/kg mitigated vincristine-induced painful neuropathy comparably to pregabalin in all tests conducted. Capparis spinosa has been previously documented to exhibit promising pharmacological effects, including pain relief in conditions such as osteoarthritis and rheumatoid arthritis (17, 32). The analgesic and anti-inflammatory properties of various parts of the plant (aerial parts, fruit, leaves, and root) have been reported, suggesting that these effects may be attributed to the presence of bioactive compounds like alkaloids, saponins, and particularly the synergistic actions of flavonoids and phenolic acids (33, 34). Earlier studies have demonstrated that bioflavonoids from the fruits of caper inhibit NF-κB, leading to reduced cytokine gene expression and survival of activated T cells (35, 36). Recent phytochemical research on flavonoids and total phenols has identified significant amounts of both compounds in the hydroalcoholic extract, aligning with the findings of previous studies (37).
Resveratrol, a prominent flavonoid, has been shown to alleviate symptoms of neuralgia by reducing apoptosis, inhibiting inflammatory responses, diminishing the expression of IL-1, and relieving oxidative stress in a rat model of paclitaxel-induced neuropathic pain (38). Additionally, other studies have found that quercetin and rutin (a flavonoid and its glycoside derivative) prevent CIPN induced by oxaliplatin through the mitigation of oxidative stress damage and a reduction in iNOS expression, effects that were observed in flavonoid-treated groups (39). Previous research has demonstrated that co-treatment with quercetin and a heme oxygenase-1 (HO-1) inducer activates the Nrf2 (nuclear factor erythroid 2-related factor 2) pathway, enhancing the analgesic effects of opioids and cannabinoids via the activation of sensitive potassium channels in a model of complete Freund's adjuvant-induced pain (40). Conversely, pretreatment with quercetin increases thresholds for heat hyperalgesia and mechanical allodynia in paclitaxel-induced peripheral neuropathy in rats and mice and reduces the elevated expression of PKCε and TRPV1 in the spinal cord, which is believed to be a potential mechanism through which pregabalin alleviates neuropathic pain (41). The capacity to activate these analgesic pathways without the significant side effects associated with opioids presents a promising approach to pain reduction.
Serotonin (5-hydroxytryptamine, 5-HT) plays a crucial role as a nociceptive transmitter both centrally and peripherally, and it has been observed that an increase in the bioavailability of 5-HT at the synaptic terminal reduces allodynia and hyperalgesia (42). In this study, cyproheptadine, a potent 5-HT2A receptor antagonist with anti-serotonergic effects, was utilized to explore the potential involvement of the serotonergic system in the effects of the caper hydroalcoholic extract (43). The investigation into the mechanism of action of the extract through the serotonin and opioid systems in ameliorating neuropathy induced by vincristine revealed that the effect of the extract at a dose of 400 mg/kg was significantly reversed by naloxone, an opioid receptor antagonist, in the hot plate and grip strength tests. Additionally, the effects of the extract in the hot plate, grip strength, and von Frey tests were notably reduced by cyproheptadine, a 5-HTP receptor antagonist. However, neither naloxone nor cyproheptadine could reverse the extract's effect in the open field and footprint tests. These findings indicate that the anti-nociceptive effect of the hydroalcoholic extract of caper at a dose of 400 mg/kg is partly mediated through both the opioid and serotonergic systems.
However, the elucidation of the mechanism of action in mechanical allodynia and locomotor impairment tests remains controversial due to the absence of a reversal effect by naloxone and cyproheptadine, except in the grip strength tests, indicating the need for more comprehensive studies. As previously noted, flavonoids and phenols have been identified in numerous studies as potent anti-nociceptive agents, with some exerting their effects through binding to opioid receptors (44, 45). Therefore, the observed improvement in peripheral neuropathy and the anti-nociceptive properties exhibited by the hydroalcoholic extract of Capparis spinosa fruit in this study may be attributed to the presence of phenolic compounds and potentially a synergistic action from the combination of other constituents within the extract.
5.1. Conclusions
In conclusion, this study has demonstrated that the hydroalcoholic extract of Capparis spinosa offers potential therapeutic benefits in treating vincristine-induced neuropathy in rats. The flavonoids and phenols within the extract appear to play a significant role in its efficacy against peripheral neuropathy. The potential engagement of opioid and serotonergic systems in mitigating pain and locomotor impairment is suggested. The findings from this research may pave the way for developing new strategies for managing chemotherapy-induced peripheral neuropathy.