1. Background
Despite significant progress in the treatment and management of myocardial infarction (MI), the mortality rate remains high (1). Myocardial infarction occurs due to an imbalance between cardiac oxygen demand and the heart's inability to supply oxygen. In this situation, metabolic and ionic imbalances in myocardial cells and mitochondrial dysfunction play a key role in apoptosis and cardiac necrosis (2). Myocardial infarction can lead to significant changes in the patient's hemodynamics, and death may eventually occur (3). Some compensatory pathways are activated to improve heart function and reduce MI-induced damage. One of these pathways involves the replacement of damaged myocardial tissue with fibrotic tissue due to the presence of fibroblasts and myofibroblasts. Although this change is initially beneficial and prevents ventricular wall rupture, its continuation causes structural changes in the heart that eventually lead to heart dysfunction and the appearance of arrhythmias. Consequently, heart failure (HF) may occur (4, 5). Alterations in blood pressure are essential factors in myocardial infarction. Studies show that MI reduces cardiac output and blood pressure. Although it improves over time, the average blood pressure level in individuals with MI is generally lower than that in healthy individuals (6, 7).
In general, MI can be divided into STEMI (ST elevation) and NSTEMI (without ST elevation). ST-segment elevation in the patient's ECG can indicate myocardial infarction, but this is not true for all patients (2). In addition to these changes, R wave amplitude can also have a diagnostic aspect. A decrease in the R wave indicates acute myocardial infarction (8). Therefore, the use of electrocardiogram as a tool to diagnose myocardial infarction is helpful.
Today, the use of herbal medicines has grown significantly compared to synthetic medicines. Herbal medicines seem to be beneficial to patients both economically and therapeutically (9). Therefore, medicinal plants can be used as adjunctive therapy in cardiovascular diseases. Lepidium draba L. (Cruciferae), a native plant to southwestern and Central Asia, contains high amounts of sulforaphane (7), which has various effects, including anticancer effect, through the induction of phase 2 protein, and the antimicrobial application of sulforaphane is also reported (6). In addition, the antioxidant and anti-inflammatory effects of L. draba extract have been reported because of its flavonoids and phenolic acids, which exist in great amounts in this plant (10, 11). According to the reports in scientific resources, aerial parts of L. draba contain different classes of compounds, such as alkaloids, terpenoids, tannins, saponins, Leuco anthocyanins, triterpenoids, and flavonoids (12). Previously in a published article in 2018 carried out by Mahomoodally et al., LC-ESI-MS/MS analysis of the methanol extracts of Cardaria draba (synonym: Lepidium draba L.) presence of some major phenolic compounds known as verbascoside, chlorogenic acid, apigenin (7)- glucoside, and 4-hydroxybenzoic acid, together with hesperidin, hyperoside, and chlorogenic acid in fewer content value plus vanillin and rosmarinic acid were reported in the plant (13). Elsewhere in another study Najim et al. reported L. draba contained many types of flavonoids including rutin, qurecetin, kampferol and luteolin (14).
2. Objectives
This is the inaugural study to investigate the effects of L. draba on cardiovascular diseases, including MI, thereby aiming to elucidate the potential protective effects of L. draba against isoproterenol-induced myocardial infarction.
3. Methods
3.1. Plant
Lepidium draba was harvested from the north of West Azerbaijan Province in June 2020. Following the drying of plants in the shade, they were pulverized and used for further experimental analysis. The collected plant was authenticated by a pharmacognosist, and the sample was kept in the faculty’s Herbarium (No: TBZFPH1989).
3.2. Preparation of Plant Extract
The dried powdered flowering branches of L. draba were defatted with 5 L n-hexane, followed by maceration with 70% ethanol for 5 consecutive days, and then filtered with Whatman filter paper. The yielded hydroalcoholic extracts were solvent-evaporated up to complete dryness via a rotary evaporator (Heidolph, Germany) at 40°C under a vacuum.
3.3. Assessment of Phenolic Profile
3.3.1. Total Phenolic Content
To determine the total phenolic content of the L. draba extract, the Folin-Ciocalteu (FC) method was used (15). Briefly, 5 mL of Folin-Ciocalteu reagent 10% (v/v) was mixed with 4 mL of Na2CO3 solution, and 0.5 mL of plant extract (1 mg/mL) was placed at 20 - 22°C for 15 min in darkness. Then, the absorbance was recorded at λmax 760 nm. Finally, the calibration curve was depicted using gallic acid (10 - 100 µg/mL) in the same process, and the obtained values were calculated as gallic acid equivalents (GAE)/g of the extract.
3.3.2. Total Flavonoid Content
The AlCl3 reagent was used to determine the total flavonoid content of the L. draba extract in a colorimetric approach (16). For this purpose, 1 mg/mL of hydroalcoholic extract of L. draba was blended with 2 mL of AlCl3, and then the absorbance was measured spectrophotometrically at λmax 415 nm. Finally, the calibration curve was depicted using quercetin (10 - 100 µg/mL) in the same process, and the obtained values were calculated as quercetin equivalents (QE)/g of the extract.
3.4. Determination of in Vitro Antioxidant Activity
3.4.1. DPPH Radical Scavenging Assay
This assay was performed as previously reported (17-19). Two mL of 0.08% freshly prepared DPPH solution was mixed individually with 2 mL of varied concentrations of L. draba extract. The ensuing solutions were kept for 30 min at 25°C in darkness. The same procedure was applied for quercetin as a positive reference compound. A spectrophotometer (Shimadzu, Japan) was used to measure the absorbance of each test solution at 517 nm. Ultimately, the inhibition percentage (I %) of DPPH free radical scavenging potential of the extract and quercetin were both computed via the subsequent formula: I % = {(absorbance of the blank - absorbance of the sample)/absorbance of the blank} × 100. Overall, the IC50 values, indicating the sample concentration that inhibits the DPPH free radicals at 50%, were estimated for extract and quercetin, correspondingly.
3.4.2. FRAP Assay
The Ferric reducing antioxidant power assay was carried out as stated by the method reported by Bardakci et al. (20). 10 μL of different concentrations of L. draba extract were individually mixed with distilled water (30 μL) and FRAP reagent (260 μL) comprising acetate buffer and FeCl3. The final solutions were kept for 30 min at 37°C. Later on, the achieved color absorbance was read at λmax 593 nm. The calibration curve was depicted using FeSO4 in the same process, and the obtained values were calculated as FeSO4 conforming to 1g of the extract.
3.5. Animal
Thirty male Wistar rats were used in this study. The rats weighing 250 ± 20 g were kept under standard circumstances (22 ± 2°C and a 12 - h light/12 - h dark cycle) in the Animal House of Urmia Medical Sciences University. All protocols were done in accordance with the Ethics Committee guidelines of Urmia University of Medical Sciences (code: IR.UMSU.REC.1399.312).
3.6. Experimental Protocol
The study was conducted in 2 days, and 5 groups were used in this study as follows: Group I (control) was left untreated during the study. Rats in group II (MI) received normal saline intraperitoneally (IP) and also isoproterenol (100 mg/kg, sc, for 2 consecutive days) to induce MI. Groups III-V were given hydroalcoholic extract of L. draba (50, 250, and 500 mg/kg respectively) for 2 days and were subcutaneously (sc) injected with isoproterenol at an interval of 24 h for 2 consecutive days.
3.7. Electrocardiogram and Hemodynamic Measurement
A Ketamine (60 mg/kg)/ xylazine (10 mg/kg) mixture was used for the induction of anesthesia. Then, lead II was used to record electrocardiographic parameters. For hemodynamic measurement, an incision was made in the midline of the animal's neck, and after isolating the left carotid, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), mean arterial pressure (MAP), and arterial Blood pressure (ABP) were measured using a polyethylene cannula. (Powerlab system, AD Instruments, Australia) (21).
3.8. Histopathological Study
For histopathological examination, cardiac apex samples were placed in 10% formalin and then stained with trichrome gomori. Finally, they were evaluated by 2 trained persons (22).
3.9. Hypertrophy
The rats were euthanized by an overdose of anesthetics, and the hearts were rapidly excised and weighed. The degree of hypertrophy was measured by the heart-weight-to-body weight ratio (g/kg) (22).
3.10. LDH Measurement
At the end of the experiment, blood samples were collected from the portal vein, and serum was used to measure LDH levels. The levels of serum LDH as a marker of tissue injury during MI were determined using enzymatic kits from Bayerpaul, Iran. The results were expressed as units of LDH present per liter (U/L).
3.11. Statistical Analysis
All data were provided as means ± standard error of the mean. To compare the mean values between groups, one-way ANOVA and the Tukey post-test were used. P < 0.05 was regarded as statistically significant.
4. Results
4.1. Phytochemical Analysis
The results of the phytochemical analysis for the hydroalcoholic extract of L. draba are shown in Table 1. The total flavonoid and phenolic contents of the L. draba extract were determined to be 92.30 mg GAE/g and 60.68 mg QE/g of the extract, respectively. Furthermore, the IC50 values in the DPPH assay for the extract and quercetin were found to be 304.45 µg/mL and 4.5 µg/mL, respectively. In the ferric-reducing antioxidant power assay, the extract exhibited a value of 0.33 mg FeSO4/g of the extract.
| Variable | Total Phenolic Content (amg GAE/g) | Total Flavonoid Content (amg QE/g) | DPPH Scavenging Activity (µg/mL) | FRAP Activity (amg FeSO4/g) |
|---|---|---|---|---|
| L draba Extract | 92.30 ± 1.84 | 60.68 ± 1.86 | 304.45 ± 10.19 a | 0.33 ± 0.008 |
a Data are presented as the mean of triplicates ± SD.
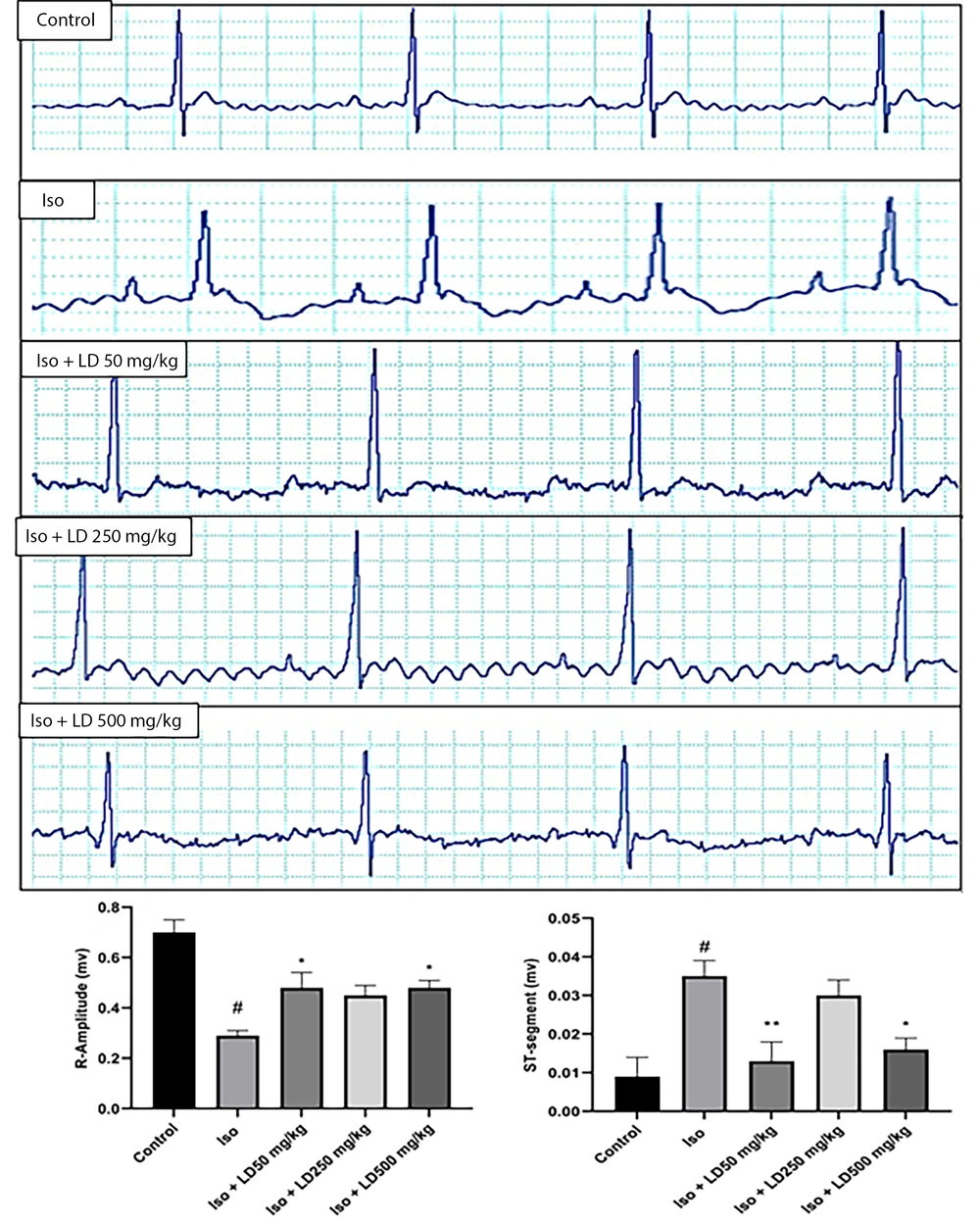
4.2. Hydroalcoholic Extract of L. draba Enhances Electrocardiogram Pattern
The electrocardiogram revealed a normal pattern in the control group, while the Iso (MI) group exhibited an ST-segment elevation (P < 0.01), indicative of myocardial infarction. Additionally, the R amplitude in the group receiving only isoproterenol injections was significantly reduced (P < 0.001) compared to the control group. The administration of the hydroalcoholic extract of L. draba led to an improvement in the ECG pattern, approaching normal levels (Figure 1A). As depicted in Figure 1B, the plant extract at doses of 50 and 500 mg/kg effectively reduced ST-segment elevation (P < 0.01 and P < 0.05, respectively) and significantly increased R-amplitude (P < 0.05) in comparison to the Iso group.
4.3. Hydroalcoholic Extract of L. draba Improved the Hemodynamic Parameters
According to hemodynamic results, the MAP reduced from 138 ± 4 mmHg in the control group to 91 ± 8 mmHg in the Iso group (P < 0.001). Hydroalcoholic extract of L. draba at all three doses significantly improved MAP (P < 0.05). Furthermore, the heart rate of rats in the Iso group was higher than the control group (P < 0.001), while treatment with hydroalcoholic extract of L. draba at all three doses of 50, 250, and 500 mg/kg decreased (P < 0.001) as compared to Iso group (Table 2).
| Groups (N = 6) | ABP (mmHg) | Systolic Pressure (mmHg) | Diastolic Pressure (mmHg) | Mean Arterial Pressure (mmHg) | Heart rate (bpm) |
|---|---|---|---|---|---|
| Control | 155 ± 1 | 138 ± 4 | 129 ± 4 | 138 ± 4 | 247 ± 10 |
| Iso | 95 ± 8 b | 91 ± 8 b | 89 ± 9 b | 91 ± 8 b | 400 ± 17 b |
| Iso+LD 50mg/kg | 118 ± 4 c | 116 ± 3 c | 113 ± 3 c | 116 ± 3 c | 287 ± 17 d |
| Iso+LD 250mg/kg | 116 ± 1 c | 116 ± 1 c | 114 ± 1 c | 116 ± 1 c | 298 ± 15 d |
| Iso+LD 500mg/kg | 124 ± 12 c | 114 ± 12 c | 109 ± 10 c | 114 ± 12 c | 238 ± 16 d |
a Values are reported as mean ± SEM.
b P < 0.001 vs. control group
c P < 0.05 vs. Iso group.
d P < 0.001 vs. Iso group.
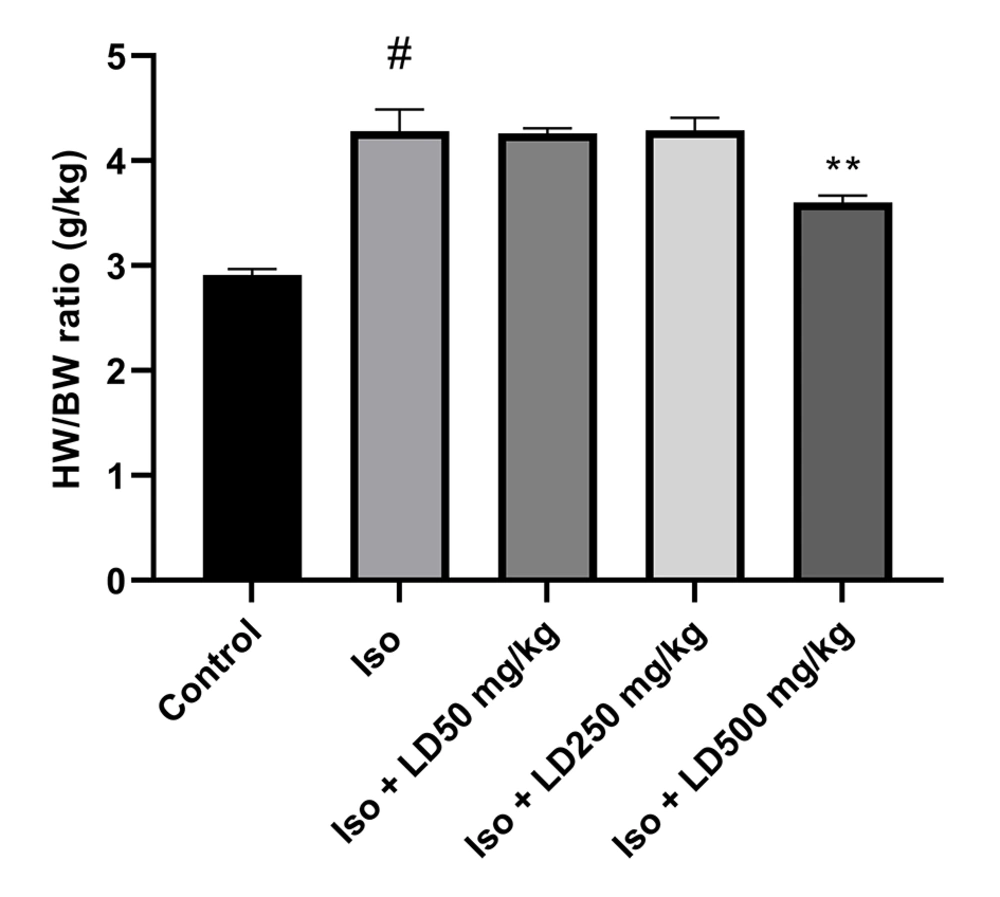
4.4. Effects of the Hydroalcoholic Extract of L. draba on Cardiac Hypertrophy
The results indicated that the heart weight to body weight (HW/BW) ratio was significantly higher in the Iso group (P < 0.001) compared to the control group. Rats treated with the hydroalcoholic extract of L. draba at a dose of 500 mg/kg during the treatment period experienced a significant reduction in the HW/BW ratio (P < 0.01) compared to the group injected with isoproterenol (Figure 2).
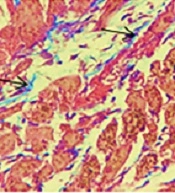
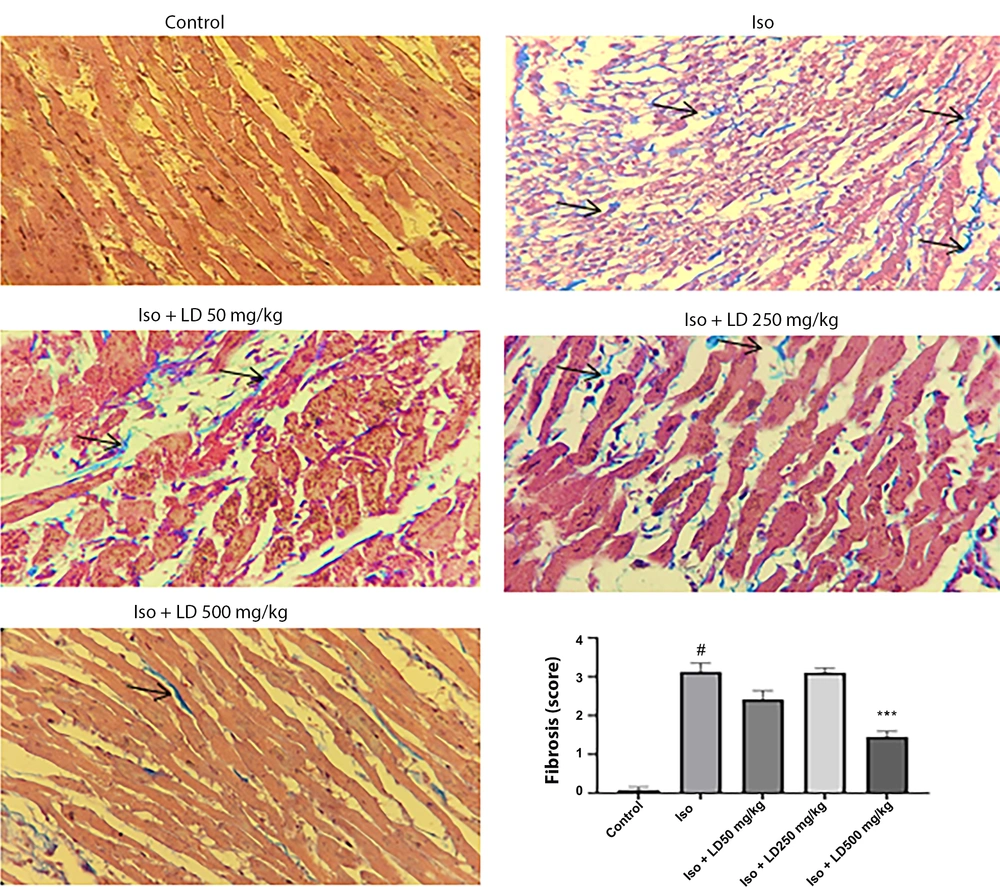
4.5. Histopathological Examination
Histological sections of the hearts from rats administered isoproterenol injections, stained with Gomori's Trichrome, showed significant sub-endocardial fibroblastic hyperplasia and severe fibrosis (Figure 3). The results indicated that treatment with the hydroalcoholic extract of L. draba (500 mg/kg) significantly reduced fibrosis (P < 0.001) induced by isoproterenol in heart tissue.
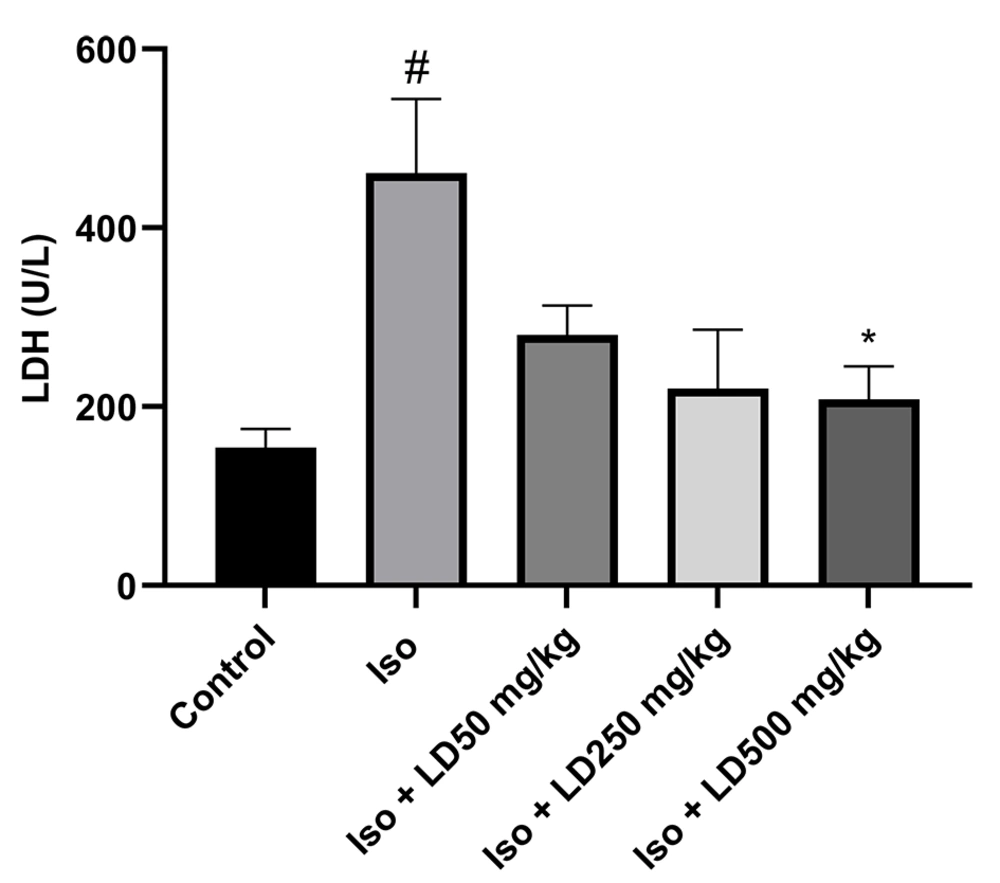
4.6. Effects of L. draba on Lactate Dehydrogenase Level
Isoproterenol injection increased LDH level from 154.95 ± 21 U/L in the control group to 461.66 ± 183 U/L in the Iso group (P < 0.05). However, L. draba administration significantly reduced the level of the serum LDH compared to the infarcted rats, who received isoproterenol only (Figure 4).
5. Discussion
The present research aimed to elucidate the potential protective effects of the L. draba extract on hemodynamic and ECG parameters, as well as heart tissue fibrosis and LDH levels following MI in rats. Our findings demonstrate that acute administration of a hydroalcoholic extract of L. draba could protect against isoproterenol-induced myocardial infarction.
Lepidium draba is a plant of the Brassicaceae family that contains several compounds, such as glucosinolates and other compounds resulting from its degradation, with beneficial effects in preventing several diseases (23). Phytochemical studies have shown that aerial parts of L. draba contain sulforaphane and erysoline (13). It has been reported that glucosinolate can reduce the size of the infarcted area in myocardial tissue and also reduce apoptosis of cardiomyocytes (24). Our results show that L. draba extract reduced histopathological changes such as fibrosis, which can be partially attributed to glucosinolate in the plant. Additionally, the presence of compounds such as alkaloids and glucosinolates can also be detected in Lepidium meyenii, another member of the Brassicaceae family (25). Overall, there are few studies with L. draba, but studies on other plants of this family, including Lepidium sativum, indicate that polyphenols are present in large quantities in this plant (26). A study by Cheng et al. (27) demonstrated that polyphenols can improve the diastolic and endothelial function of the heart in people who have experienced myocardial infarction. Polyphenols also have protective effects in cardiac ischemia through their capability of decreasing oxygen free radicals (28). The findings of the phytochemical analysis of the L. draba hydroalcoholic extract revealed moderate antioxidant potential in DPPH radical scavenging activity, along with measurable ferric ion-reducing activity, which is mostly connected to the polyphenols and flavonoids content in the hydroalcoholic extract.
Several studies have indicated that isoproterenol, a synthetic β-adrenoceptor agonist, is used to induce MI in rats (29, 30). Studies show that isoproterenol-induced MI can be a valid and reproducible way to evaluate new cardioprotective drugs (31). We found that hemodynamic factors, such as mean arterial pressure, decreased in the MI (Iso) group, and administration of L. draba extract improved these parameters. It was also observed that heart rate improved and decreased in the group treated with L. draba extract. These positive effects on hemodynamic parameters can be partially attributed to the polyphenols present in the plant.
A decrease in R wave amplitude was observed in rats receiving isoproterenol. ECG analysis showed reduced R wave amplitude in the Iso group, but acute administration of L. draba extract in high doses increased the R wave amplitude, which can be related to the reduction of edema in the heart tissue (32, 33). Consistent with our results, Rajadurai and Stanely Mainzen Prince (34) reported that isoproterenol-induced MI can lead to ST-segment elevation on the ECG, which is an important sign of the presence of myocardial ischemia. Acute administration of L. draba extract at high doses reduced ST-segment elevation. Elevation of ST-segment on ECG pattern occurs due to a potential imbalance in the boundary between ischemic and non-ischemic regions, while R-amplitude decreases because of the presence of edema in myocardial tissue as a result of infarction induced by isoproterenol injection (35). It was also proved that elevation of the ST-segment may be a sign of necrosis of the myocardial tissue (36). Hypertrophy caused by isoproterenol can be due to increased cell death, fibrosis, and abnormalities in normal heart function. Treatment with hydroalcoholic extract of L. draba reduces cardiac hypertrophy, which can be considered a cardioprotective mechanism (37, 38).
Metabolic damage or myocardial infarction causes a large number of diagnostic factors to leak into the extracellular space (39). The presence of cytosolic enzymes such as lactate dehydrogenase (LDH) is considered a diagnostic marker when tissue is damaged. The amount of these enzymes in serum can vary due to changes in membrane permeability or rupture (35). Lepidium draba extract-treated groups demonstrated less activity of LDH in the serum, indicating that the extract maintains cell permeability and prevents the leakage of these enzymes. It was reported that sulforaphane present in the plant can reduce the level of serum LDH activity caused by tissue damage (40), which is in line with our findings.
5.1. Conclusions
Acute administration of the hydroalcoholic extract of L. draba plays a protective role in myocardial infarction and could be considered an adjunctive treatment option for myocardial infarction.