1. Context
Transdermal patch systems (TPS) are self-contained and discrete drug delivery systems that deliver drugs directly into the systemic circulation. The first TPS was approved in the early 1970s, advancing research efforts and leading to greater use by pharmaceuticals. Transdermal patch systems have superior stability to other transdermal drug delivery systems (TDDS), which helps to prolong drug delivery for disorders that require long-term treatment. Transdermal patch systems have several evident benefits over other delivery methods, including avoiding first-pass metabolism, consistent distribution of therapies to the systemic circulation, improved patient compliance, dosage intervention, avoidance of frequent dosing, overcoming drug and enzymatic degradation, and controlled release of the drug. Despite these appealing benefits, TPS remains in the market with only a few commercial products licensed by the United States Food and Drug Administration (USFDA) in therapeutic areas, including smoking cessation, hypertension, hormone replacement therapy, cardiovascular problems, contraception, pain, and neurological disorders (1-3).
From the early stages of the development of transdermal patches, which was the Anointed applied bandaged rubbed to the skin in 1550 BC until the present generation Sumatriptan developed by Zecutiy®, TPS growth remains interesting. Miller says he spends more of his adult life designing transdermal patches by dealing with issues to get a well-defined profitable product. He also gave his view that a transdermal patch is an engineered, external, continuous, and long-acting dosage form where no other formulations have all of these included benefits (4).
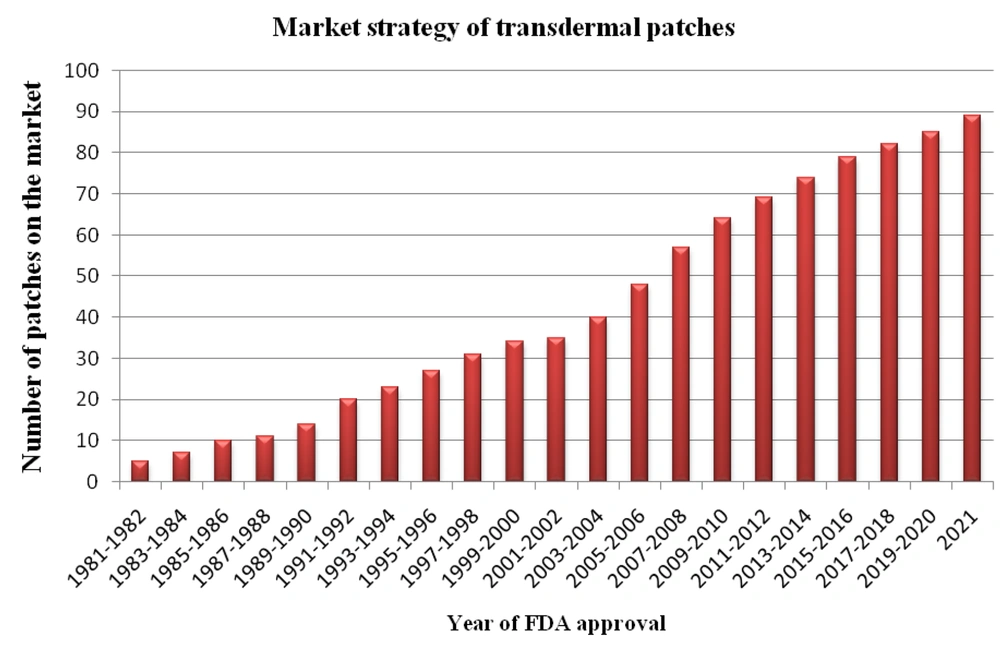
Over the past years, the new transdermal delivery system manufacturing rate has increased by more than thrice, approximately once in 7.5 months. According to the statistics, over one million transdermal patches are manufactured annually. The number of transdermal patches in the market approved each year (5-7) by the FDA is depicted in Figure 1.
According to USFDA enforcement reports, a majority of high-profile pharmaceutical recalls have been noticed due to a lack of quality in drug products; out of those recalls, most are from biopharmaceutical classification system (BCS) class II and IV, which affect both public and individual patient's health (8-11). The transdermal patches, such as clonidine transdermal patch (12) and fentanyl transdermal patch (13), were recalled by the FDA due to quality issues, tabulated in Table 1.
| Company | Patch | Recalled Reason | Recalled on | Details |
|---|---|---|---|---|
| Teva Pharmaceuticals (Actavis Laboratories) | Clonidine transdermal patch (hypertension) | Failed to meet impurities and degradation specification | October 28, 2021 | 0.1 mg/day; 4 patches per carton. Lot 1369117B (Exp. 11/21) |
| Alvogen Inc. | Fentanyl transdermal patch (opioid analgesic) | Product mislabeling (12 mcg/h patches had been labeled as 50 mcg/h in the cartons) | April 19, 2019 | Lot (180060 and 180073) with an expiration date of May 2020 and June 2020 |
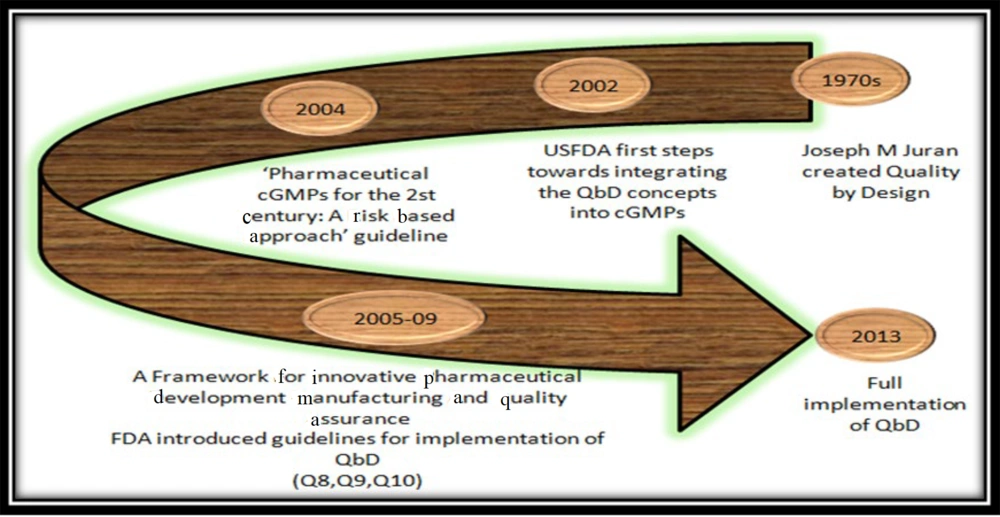
Transdermal patch systems quality issues have impacted the safety profile of pharmaceutical drugs, resulting in some product recalls and/or market withdrawals (1). More quality-linked problems arise owing to packaging errors, crystallization during warehousing, misbranding, and unfavorable effects which are interrelated to moisture penetration, dose dumping, toxicities due to excessive loading, instability of the product, and the threat of skin irritation and sensitization (1, 14, 15). With a view to potentially overcome the disadvantages and for effective product innovation, the quality by design (QbD) framework could be used (16). In the early ages of the economic revolution, in pilot plant development, the quality of the product was known only by end-product testing. We can conclude that for QbD developed by quality expert Joseph M. Juran for new and existing products to overcome all the quality issues, it took almost 50 years from the day of creation to implement QbD as a choice for industries to solve the quality issues with the help of guidelines as depicted in Figure 2 (17).
Quality by Design has received great attention recently, and pharmaceutical companies are emphasizing it more than ever before. However, a lack of knowledge of its concepts and terminology might lead to a lack of confidence in applying its principles to product development. Quality by design framework facilitates a systematic approach with pre-determined goals to start a new product, which comprises drug product performance, patient safety, and efficacy. For pharmaceutical progression, QbD aids not only in recognizing the findings of critical material attributes (CMAs) and critical process parameters (CPPs) but also in knowing their function and interaction in obtaining a target quality product. Quality by design aims to spot variables, i.e., both product and process-related, which are critical to product quality, safety, and efficacy, which further facilitates a control strategy to consistently produce drug products with desired quality characteristics (18, 19). To accomplish this goal, drug product quality attributes are effectively linked to formulation variables, such as drug substance and excipients attributes, and the manufacturing process to identify sources of variations and inherent risks in the early phases, which are to be minimized. This relationship is vital to implementing a flexible and robust manufacturing process that can consistently produce a quality drug product (18). The link between product attributes and quality has been inadequately defined in the past; the FDA has assured quality by requiring sponsors to utilize a standardized manufacturing method and imposing strict standards based on observed outputs of clinical trial batches (20). Therefore, the application of QbD methodologies from a commercial aspect is strongly advised, as it lowers costs at all phases of research and speeds up the commercialization process (21).
The standard of the pharmaceutical product is primarily achieved when patient well-being is met, and, therefore, it has just recently been revealed that even the USFDA is of the opinion that instead of quality being assessed at the end, quality should be incorporated into the product (18, 22). The following equation illustrates where quality originates:
Quality of pharmaceuticals = f (drug substance, excipients, manufacturing, packaging)
Acknowledging how formulation and manufacturing process variables affect product quality is essential, which would be the function “f” in the equation above. The ‘f’ says for achieving a quality pharmaceutical formulation, the drug substance, excipients, manufacturing process, and packaging system should be strongly high profiled (23).
The International Council on Harmonization (ICH) has developed guidelines globally to match technical requirements for the manufacture and use of pharmaceutical ingredients and drug products and promote continuous improvement within the pharmaceutical quality control systems. The ICH guidelines have facilitated the transformation of traditional, univariate testing product development techniques into multivariate, scientific, risk-based procedures led by QbD principles (24). In the 21st century, the QbD framework was adopted in the chemistry, manufacturing, and controls review system in pharmaceutical current good manufacturing practices (cGMPs) to produce high-quality pharmaceuticals (25). The QbD method was used in the production, formulation, and evaluation of drug delivery dosage forms. This method is beneficial because it aids the user in selecting component levels for a specific formulation and models the output (response) using various polynomial equations. In other words, the formulator can forecast formulation compositions that are related to the desired performance (26).
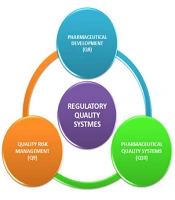
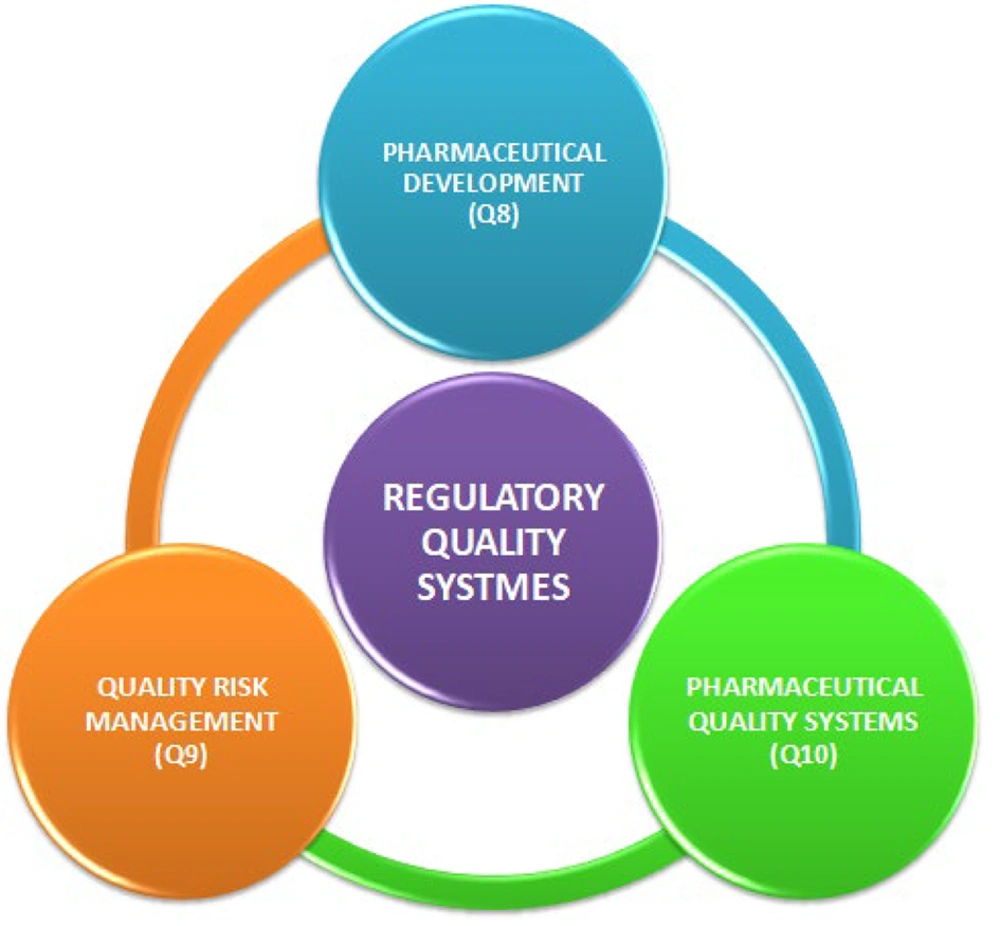
Pharmaceutical development (ICH Q8), quality risk assessment (ICH Q9), and pharmaceutical quality systems (ICH Q10) are the ICH guidelines that summarize the strategy for attaining product performance using QbD in the FDA guidelines (Figure 3) (27).
The desired outcomes of a successful QbD program are as follows (28, 29):
- Built-in product quality (for efficacy and safety)
- Renders huge cost benefits
- Establishes operating design space and fringe of failure
- Enhances manufacturing process understanding and control strategy
- Enhances process capability and robustness
- Facilitates post-approval regulatory oversight
- Helps in gaining more opportunities for regulatory approaches
- Incorporates risk management
- Holistic approach - applicable to all aspects of pharmaceutical development
This will help pharmaceutical companies to improve the quality of their drugs. Therefore, the QbD method was established to streamline the research and development (R&D) process, increase process knowledge, and control capabilities to relieve and eventually solve this difficulty (30). A variety of case studies for the implementation of QbD in the development of numerous pharmaceutical products have been published in the literature. However, there is a lack of extensive reviews on QbD-based development in TPS (14). This review attempts to describe key factors involved in the development of TPS using the QbD approach.
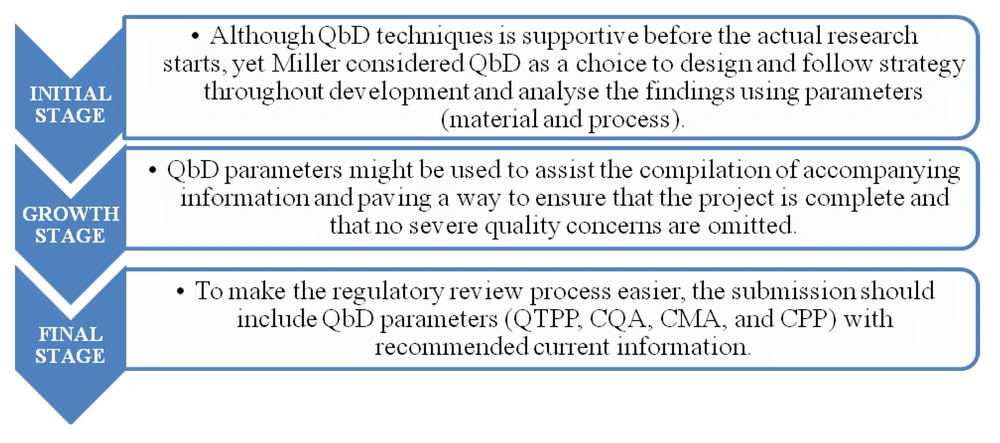
Miller suggested three ways to implement QbD techniques into the formulation of a novel transdermal product (31), which is depicted in Figure 4.
2. QbD Implementation for TPS
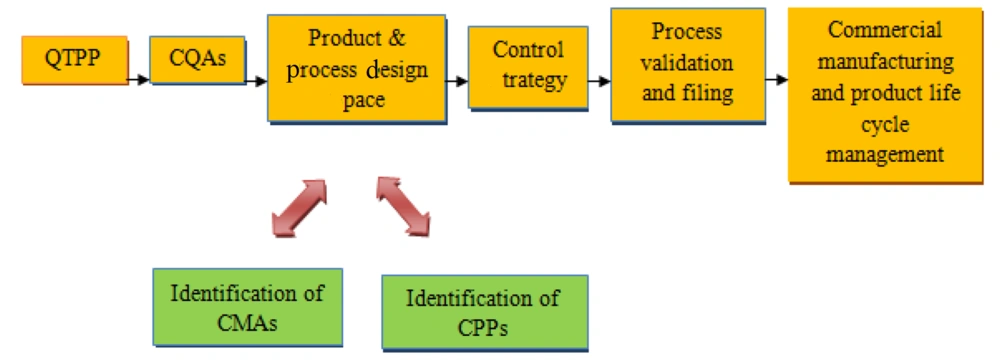
The American Association of Pharmaceutical Sciences (AAPS), the USFDA, and the United States Pharmacopeia (USP) together conducted a meeting in 1997 to examine the regulatory rules regulating the development of transdermal drug products (32). By defining the quality target product profile (QTPP), we will determine the potential critical quality attributes (CQAs) and link the CMAs and CPPs to perform the risk assessment analysis, thereby reaching out to design space, the control strategy is implemented; finally, the product life cycle is monitored for continual improvement. Quality by design has recently gained popularity in industrial pharmaceutics since it saves costs and time, especially when scaling up (33). The process flow of QbD implementation is depicted in Figure 5. To develop TPS products with acceptable CQAs, systematic risk assessments, and QbD characterizations can also aid in the selection of appropriate controls for manufacturing process variables (27).
3. QTPP for Transdermal Patch Systems TPS
The concept of QTPP establishes the basis for product design, which is considered new in the QbD concept. The QTPP is a “prospective description of the quality characteristics of a therapeutic product that should ideally be reached to assure the intended quality, taking into consideration the drug product's safety and efficacy” (22). A QTPP is generated using desired labeling information and specifies strength, indication, contraindication, dosage form, dose, frequency, and pharmacokinetics (34). In addition, marketing suitability, the dosage form, method of administration, residual drug, safety advice, and containers and sealing systems for the target profile might all be included in the QTPP for a drug product.
The QTPP should be developed as soon as the drug candidate is selected, and it is widely recognized as critical for establishing a strategic framework for product development. The main aim and efficient product/process design would result only from predefined product quality specifications (14). The elements of QTPP and their justification are shown in Table 2.
| QTPP Elements | Goal | QTPP of TPS with Justification |
|---|---|---|
| Dosage form | Transdermal patch | Systemic distribution of medicine over a long period through skin penetration. |
| Route of administration | Transdermal | Peripheral adverse reactions are avoided through transdermal delivery. |
| Dosage strength | Less than 25 mg | Controlled drug release |
| Pharmacokinetics | Bioavailability requirement | Drugs are absorbed and distributed properly in the body. |
| Shelf life | When kept at room temperature, it lasts at least 24 months. | Assures the safety and quality of the product. |
| Drug product quality attributes | ||
| Physical properties | Comply with regulatory requirements: Must adhere to the same compendial or other applicable requirements. | During shipping and warehousing, differences in drug particulate matter, morphology, structure, and crystallization might occur, which influences the end product's stability and efficiency. |
| Appearance | ||
| Particle size | ||
| Polymorphism | ||
| Thickness | ||
| TPS performance | Ensure good adherence and ease of removal from the surface of the dermis with no adhesive residue. | |
| Adhesion | ||
| Ease of removal | ||
| Identification | Verification procedures should be matched to the drug product, the presence of the drug in the final product should be validated, and distinct compounds with similar structures should be distinguished. | |
| Assay | To develop a dosage that will assure medication bioavailability and increase clinical effectiveness. | |
| Content uniformity | The consistency of the product will influence the stability and effectiveness of the medication. | |
| Drug depletion/release rate | In vitro and in vivo tests will be verified, and the active pharmaceutical distribution will be observed. | |
| pH | Generally, the formulation's pH should match the skin's biological pH because skin soreness and swelling will occur if the pH is not balanced. | |
| Skin condition | ||
| Skin irritation | The patches on the skin should cause only the least amount of or no irritation in addition to preserving the clinical efficacy. | |
| Container closure system | Avoid interaction between container and medication | To meet the intended shelf life and retain TPS consistency throughout transit, a suitable container closure method is required. |
Abbreviations: QTPP, quality target product profile; TPS, transdermal patch systems.
4. CMAs
A physical, chemical, biological, or microbiological component of an input material must be within a certain limit, range, or distribution in order for the output material to be of the required quality (22). “Material” refers to raw materials, starting materials, reagents, solvents, process aids, intermediates, and so on; however, “Attribute” refers to a material's measurable physical, chemical, biological, or microbiological quality or qualities. It is important to select an appropriate CMA from other grouped CMAs that are observed to aid in supplementing particular product development (35). The effect of CMA on CQA and their justification are tabulated in Table 3.
| Critical Material Attributes | CQAs of TPS with Justification | |
|---|---|---|
| Active pharmaceutical ingredient | ||
| Particle size/area | Drug delivery rate; drug crystallization; presence of impurities | It affects product quality, safety, and efficacy, which causes an issue in permeation to the subcutaneous membrane. Crystallization affects drug delivery. |
| Polymorphs | ||
| Impurities | ||
| Solubility in formulation matrix | ||
| Drug crystallization | ||
| Melting point | ||
| Partition coefficient | ||
| Pressure sensitive | ||
| Adhesive | Delivery rate; patch adhesion; cohesion (cold flow); irritation/sensitization; residual drug; assay/impurities | Affects adhesive properties, diffusion of drug, efficacy, and safety of the product |
| Adhesive type | ||
| Viscosity | ||
| Type/ratio | ||
| Molecular weight | ||
| Residual monomers | ||
| Excipients | ||
| Permeation enhancer | Delivery rate; crystallization; assay/impurities | Affects product stability and safety |
| Crystallization inhibitor | ||
| Rate controlling membrane | ||
| Solvent | ||
| Backing membrane | Patch integrity | Affects the product efficacy |
| Release liner | Flexibility | Affects the product efficacy |
Abbreviations: CQAs, critical quality attributes; TPS, transdermal patch systems.
5. CPPs
A CPP in pharmaceutical production for process variables that have an impact on a CQA and, therefore, should be monitored or controlled to ensure the drug product obtains the desired quality (22). It is important to select an appropriate CPP from other grouped CPPs, which is found to aid in supplementing particular product development (35). Critical process parameters that affect CQAs are tabulated in Table 4.
| Unit Operation | CPPs | CQAs | |
|---|---|---|---|
| Mixing | Adhesive, enhancer, API, and other excipients are mixed in a compounding procedure. | Product temperature; order of addition; agitation speed; agitation time | Drug identification; drug content; appearance; viscosity; particle size |
| Coating, drying, and laminating | Forming a drug-in-adhesive layer out of a liquid compound | Drying airflow; drying temperature; machine speed; laminator roll pressure; laminator roll size | Drug identification; drug content; polymorphism; patch thickness |
| Filling, laminating, and sealing | Filling a reservoir blend onto a multilayer membrane, laminating and sealing it | Fill volumes; sealing station temperature; time; pressure | Patch fill weight; seal integrity; drug identification; liquid presence |
| Die-cutting and pouching | The patch is trimmed to a specific size and form and inserted in the main package in the final stage. | Web tension; die temperature; sealing temperature; sealing time; sealing pressure | Patch shape; primary packing; output size; pouch seal integrity; drug identification |
Abbreviations: CQAs, critical quality attributes; API, active pharmaceutical ingredient; CPP, critical process parameters.
The process flow of a drug-in-adhesive transdermal patch development is as follows:
- All the ingredients are weighed and mixed as a liquid drug-containing adhesive solution
- Coating, drying, and lamination of liquid mass are carried out to obtain a drug-in-adhesive layer.
- The laminate is cut into layers.
- Punching of the patch with proper edged texture
- Patches are converted into pouches from single patches.
Producing methods are generally known to have a substantial influence on the product's qualitative elements (35).
6. CQAs
After establishing QTPP, the next step is to determine CQA for the TPS, which is “A physical, chemical, biological, or microbiological property that should be within a suitable limit, range, or distribution to provide the intended product quality,” according to ICH Q8 (22). To assess finished TPS, three types of tests are often used: Product quality testing, in vitro drug product performance tests, and in vivo drug product performance tests (1). Visual description, identification, assay, impurities, content homogeneity, residual solvent levels, polymorphism, and microbiological limitations are examples of product quality attributes for TPS. The possible influence on patient safety and efficacy is used to choose CQA from a list of quality factors. During the production process, important qualities must be assured in every single product unit. Critical CQA might be determined using product specifications and regulatory guidelines (14).
When it comes to detecting CQA from a set of TPS quality parameters, a risk-based analysis tool helps a lot. The criticality of quality characteristics, for example, would be determined by their potential influence on patient safety and efficacy (14). Some of the common and product-specific CQAs concerning TPS are tabulated in Table 5.
| Common CQA of a TPS | Product-Specific CQA of TPS | Justification |
|---|---|---|
| Assay; content uniformity; moisture content; impurities and degradation: (Residual solvents; residual monomers); In vitro test (drug release): (Drug release; drug permeation); drug polymorph; particle size; enhancers content; microbial test; compatibility | Adhesion test: (Shear adhesion; peel adhesion; TPS integrity; leakage test; cold flow property; tack test); cohesion test; patch size/shape; skin irritation; sensitive test; flatness test; water vapor permeation test; elongation | Any deviation in the evaluation of the CQA will affect the drug safety, efficacy, quality, and product stability. Risk factors must be mitigated because unintended drug releases may happen. Incidence of erythema and edema during application may also occur |
Abbreviations: CQA, critical quality attribute; TPS, transdermal patch systems.
When the relationship between QTPP and CQA in TPS for the better product requirement is correlated, it impacts the final finished evaluation test if not properly analyzed (14).
7. Design of Experiments
The design of experiments (DOE) model seems to be very useful when the combined effect of CMAs (raw material) on the quality of the product is examined. During the formulation development phase, the primary purpose is to investigate the important variables that control formulation performance and its robustness, followed by formulation optimization. The development studies incorporating DOE would assess the causes of variability (14).
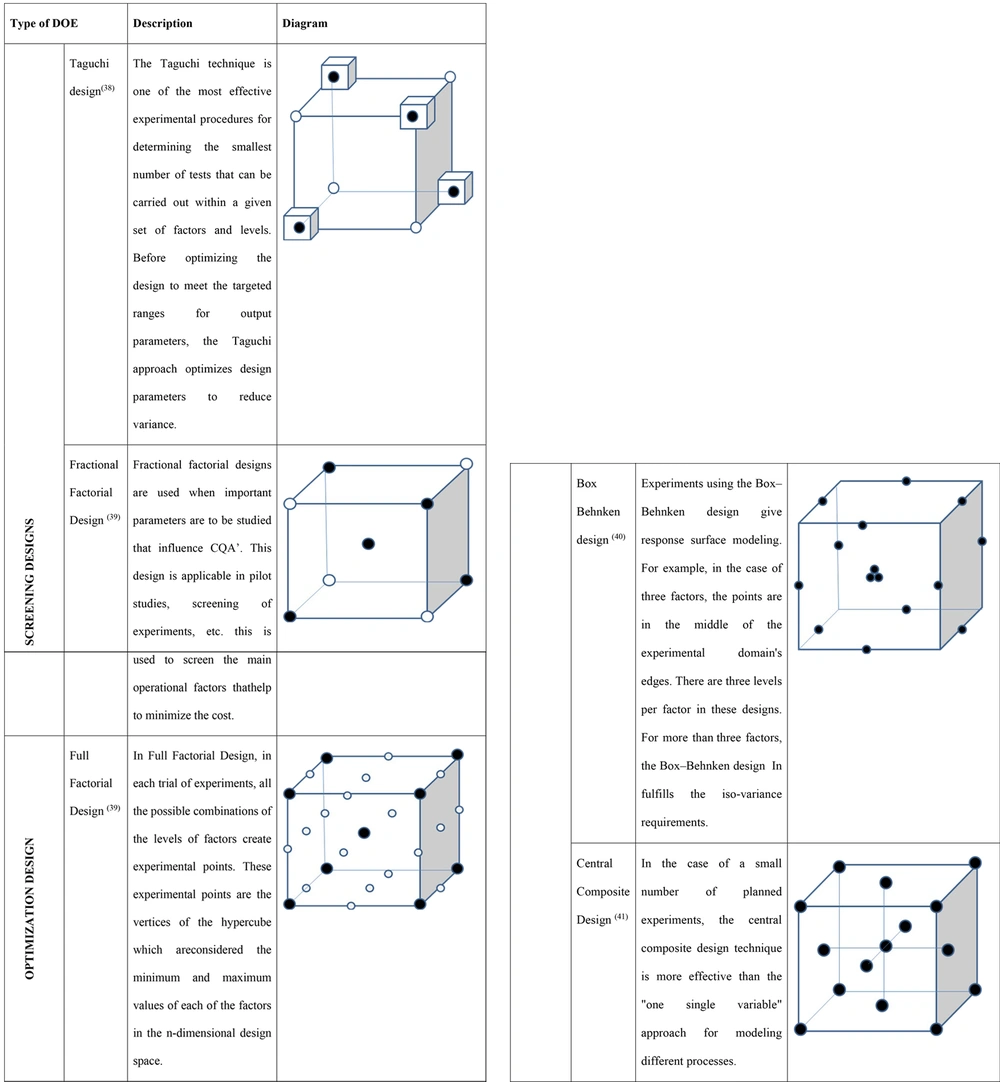
The multidimensional combination and interaction of material attributes and process parameters that have been proved to give assurance of quality are according to ICH Q8. Functioning inside the design area is not regarded as a process deviation; however, leaving the design space is a major elimination that might lead to regulatory approval revisions after the fact (36). Some of the DOEs used for screening and optimization of TPS are depicted in Figure 6 (37).
8. Risk Assessment: Linking CMAs and CPPs to the Drug Product CQAs
Risk assessment is a science-based method used in quality risk management (ICH Q9) that can assist in determining whether material attributes and process parameters might have an impact on product CQAs. Depending on the prior experimental data, risk assessment techniques might be used to determine and assess factors that could have a major impact and affect product quality.
Assessing hazards and analyzing problems are all part of the risk assessment process that is associated with TPS. It is the first phase in a three-part quality risk management approach that also includes risk control and risk evaluation. Risk management generates the decisions to limit and/or accept risks. The goal of risk management is to keep the risk at an acceptable level. Risk communication and risk management information between stakeholders (e.g., regulators and industry, industry and the patient, inside a firm, industry, or designated area) should be active throughout the risk management process. The data might be on the existence, nature, form, likelihood, severity, acceptability, control, treatment, detectability, or other elements of quality hazards (42).
Risk assessment is composed of three parts: Risk identification, risk analysis, and risk evaluation.
(1) Risk identification: Statistical data, theoretical analysis, professional viewpoints, and corporate concerns might all be used to explore potential sources of risks associated with the risk assessment.
(2) Risk analysis: The evaluation of the risk based on the identified hazards
(3) Risk evaluation: Using a statistical scale, compare the estimated risk to specific risk criteria to establish the risk's magnitude (42).
Some or all of the CQAs can be evaluated and ranked using risk assessment tools (14). Quality risk management methods and statistical tools lead to greater flexibility in the implementation of quality risk management concepts (26).
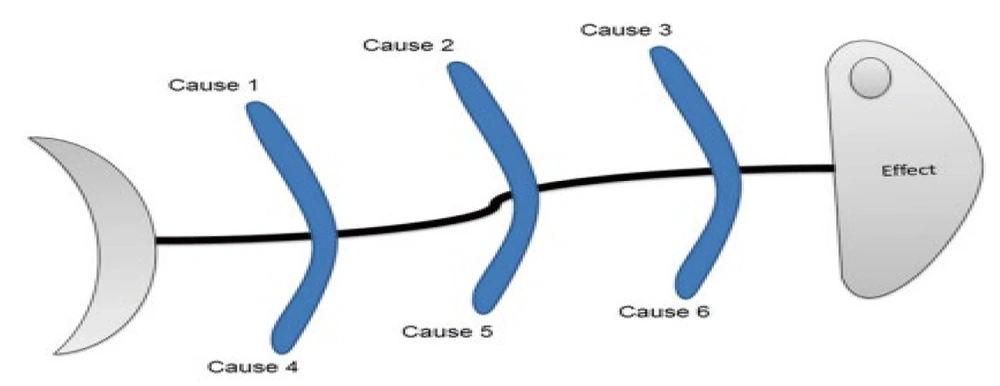
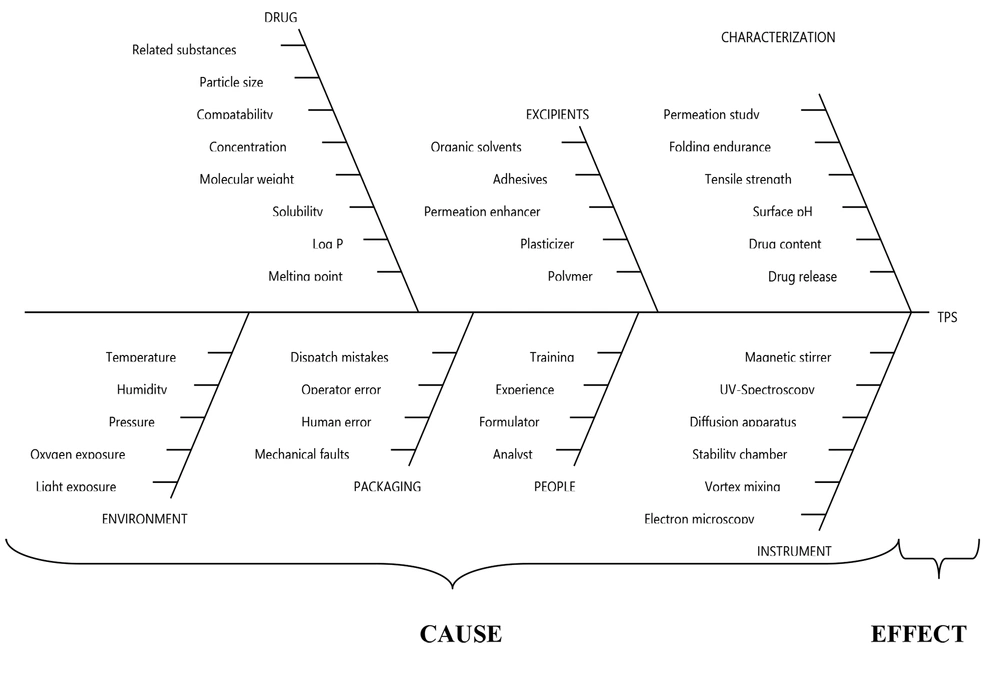
In the 1960's Kaoru Ishikawa, a Japanese professor who created quality management systems, introduced Ishikawa diagrams. It is termed a fishbone diagram due to its structure, which depicts the view of a fish skeleton, i.e., the head termed as “effect” and the bones signifies potential causes (43) as depicted in Figure 7.
The Ishikawa diagram is used before and after a problem emerges to prevent defects. It can be generally used in combination with other quality management tools, such as flow charts and failure mode and effect analysis (FMEA), which is a comprehensive procedure that frequently involves brainstorming throughout the design and manufacturing phases of a product (44).
Numerous organizations still use the Ishikawa diagram to take action after determining the root cause of a problem that impacts on the quality of the finished product. The following points will help in assessing the way how to apply the tool for examining the issues:
(1) Find the issue.
(2) Pick out the primary issues out of them.
(3) The origin of the problem will be figured out.
(4) Explore diagrams for problems (43). The application areas of the Ishikawa diagram are constantly growing and are employed in a variety of fields. It is something that aids in the drawing of the cause-and-effect diagram for acceptable limits (45).
To systematically relate the quantitative impacts of CMAs to CQAs of the final product, an initial risk assessment must be undertaken to identify CMAs, followed by the implementation of multivariate DOEs (46). Risk assessment is being used to identify weak areas that need to be investigated deeply and can be used to guide the control plan, which includes future-level changes in the development of the product (42). Ishikawa diagram for TPS illustrating their potential parameters is shown in Figure 8.
9. Risk Estimation Matrix for TPS
A risk assessment matrix is a tool for determining the probability and severity that is predicted or expected to happen. Frequent, probable, occasional, rare, and unlikely are examples of different degrees of probability. Catastrophic, critical, marginal, and negligible are some examples of severity. Activities that are both common and catastrophic are regarded as extremely high risk; nevertheless, activities that are both uncommon and insignificant are considered low risk. The matrix demands recommendations to limit or eliminate the hazards in addition to identifying them (47).
The risk priority number (RPN) is based on three important attributes: The degree of the consequence of loss, the chance of frequency, and the ability to observe each failure mode. By multiplying these three attributes according to the formula below, RPN is calculated:
RPN = S × P × D
Where S is the severity of the effect of failure, P is the probability of failure, and D is the ease of detection.
The RPN does not always contribute to selecting the right plan of action in response to failure modes; however, it will assist in identifying upper and lower limits for defining the most concentrated zones. In other words, in the analysis and corrective action, a failure mode with a high RPN number should be given the utmost priority (48).
The risk estimation matrix is tabulated based on the QTPP and CQA/CMA/CPP parameters for TPS in Table 6.
| QTPP and CQAs | Route of Administration | Dosage Form | Site of Activity | Appearance | Stability | Type of Packaging Material | Mechanical Properties of Patch for Skin Application |
|---|---|---|---|---|---|---|---|
| Physical properties | Low | Low | Low | High | High | Medium | Low |
| Homogeneity | Low | High | Low | High | High | Low | Low |
| pH | High | Medium | Medium | Low | Medium | Low | Low |
| Skin feeling | Medium | Medium | Low | Low | Low | Low | Medium |
| Drying time | Medium | High | High | Low | Medium | Medium | High |
| Stability (physical, chemical) | Low | High | Low | Medium | High | Medium | Medium |
| Stability (microbial) | Low | High | Low | Medium | High | Low | Low |
| Patch appearance | Medium | High | Low | Medium | High | Low | High |
| Patch burst strength | High | High | Low | Low | High | Low | High |
| Skin adhesion | High | High | Medium | Low | High | Low | High |
| Patch flexibility | High | High | Medium | Low | High | Low | High |
| Patch integrity | Low | High | Medium | Low | High | Low | High |
| CMA/CPP and CQA | Content Uniformity | Diffusion | Folding Endurance | Weight Variation | Thickness | ||
| Drug | High | Medium | Low | Medium | Low | ||
| Polymer | Medium | High | Medium | Medium | Medium | ||
| Plasticizer | Medium | High | High | Low | Low | ||
| Permeation enhancer | Low | Medium | Low | Low | Medium | ||
Abbreviations: CMA, critical material attributes; CPP, critical process parameter; CQAs, critical quality attributes; QTPP, quality target product profile.
10. Design Space
The next stage is to create and optimize the production process once the formulation has been refined. Once the acceptable CQAs have been determined in the design space, evaluation studies can be used to identify the acceptable variability in process parameters and material attributes (49). It is important to note that a set of normal limits based on basic testing does not create a design space; instead, the acceptable ranges must be based on multiple trials that consider the major impacts and the interactions of the process factors (36). Performing within these acceptable parameters, which are combined to constitute the process design space, ensures quality (50).
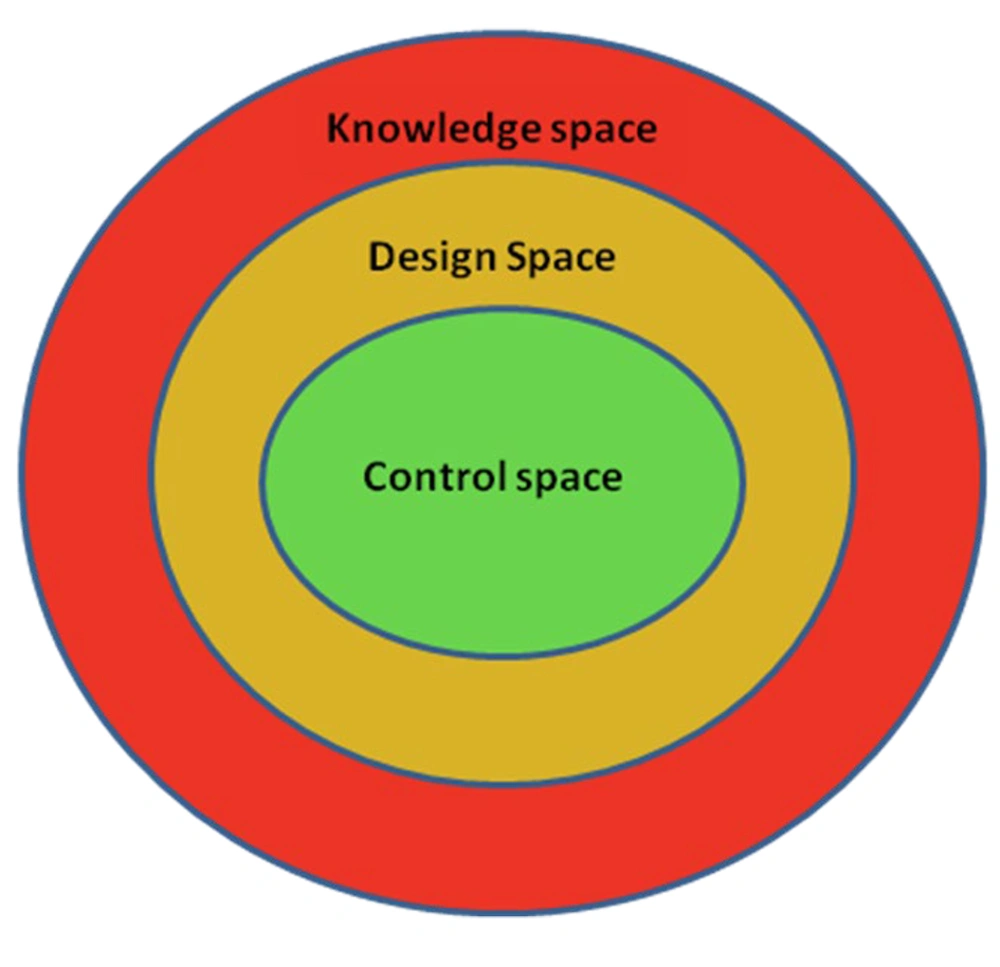
Design space is a multivariate model where process understanding is important. Regardless of how a design space is created, operating inside the design space will result in a product that meets the specified quality standards. Although design space is not a necessary component for determining the edge of failure, it might be useful to identify the point at which process parameters or material qualities fail and the appropriate quality criteria that are no longer satisfied. The use of design space can help cover the whole process, allowing for greater operational flexibility (22, 50). Control space is the region within the design space that is considered to be precise where a planned set of controls as normal operating ranges is implemented for future commercial manufacturing, and design space is a region within the knowledge space that consists of CQAs to be met for the predefined specifications. Knowledge space is a region where the CMAs and CPPs within the process are technically operated (51).
The established product design space, which is included in the regulatory document as in-process and product specifications, shows the range of acceptable product CQAs (14). There are three main regions in design space, including knowledge space, design space, and control space, illustrated in Figure 9.
11. Control Strategy
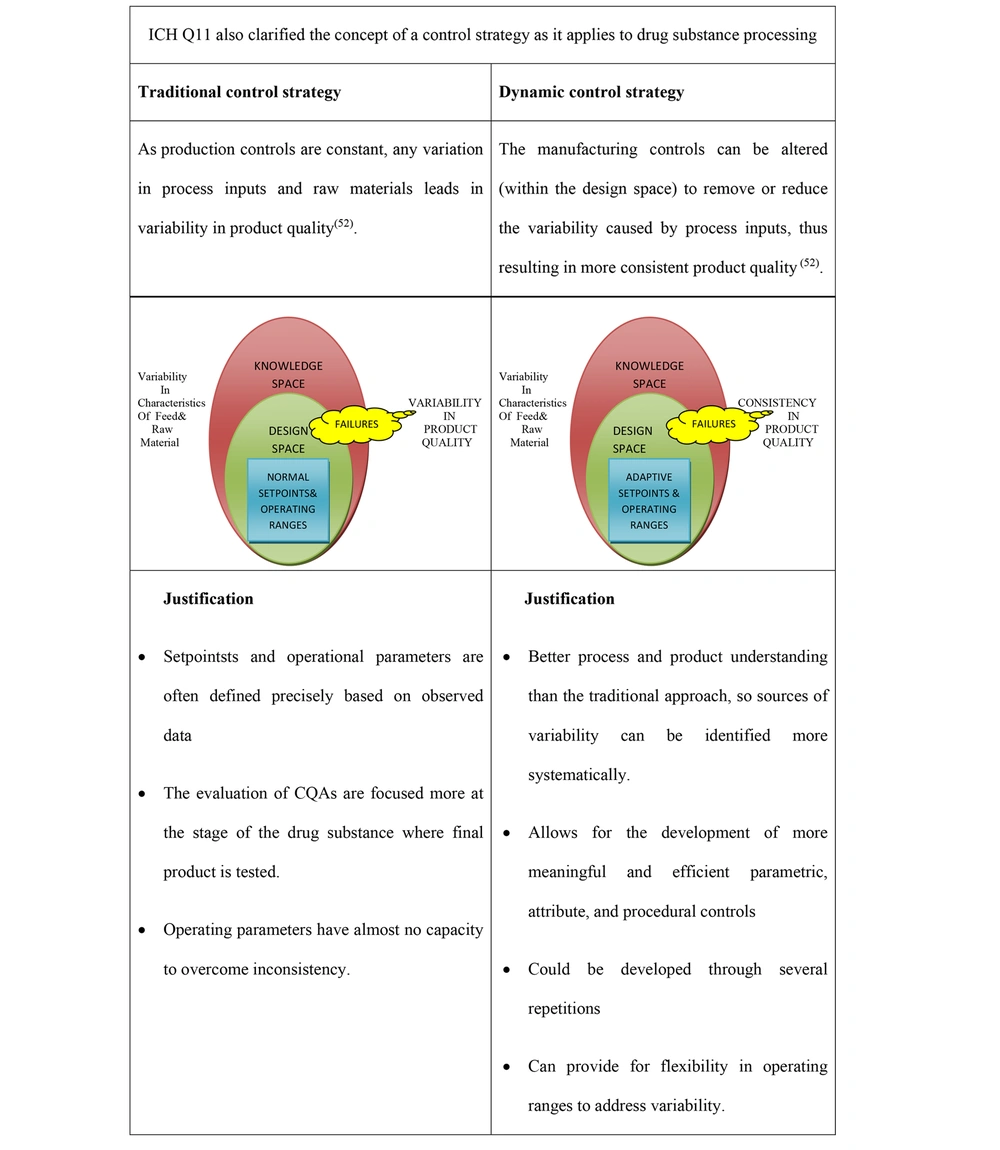
A control strategy is a set of controls generated from existing product and process knowledge that ensures product and process quality and performance. Critical material attributes and CPPs linked to the drug substance or completed product quality attributes, components, facility, finished product standards, and the accompanying procedures and monitoring frequency might all be included in these controls. Understanding the product and process, along with quality risk management, can regulate the process. Therefore, the raw material inconsistency might be adjusted to achieve consistent product quality (28). There are two different approaches to establishing a control strategy, including traditional control strategy and dynamic control strategy. The different approaches to control strategies are shown in Figure 10.
Two approaches to opting for a control strategy (52)
12. Validation and Continuous Improvement
The process will be assessed to show if it consistently provides the product with specified quality attributes when operated within the design space once the product design, process design, and suitable control strategy have been defined. Process validation further verifies if the design space was developed using a research phase pharmaceutical development report that correctly mimics the production scale process (14).
Once the product has been validated, CQAs must be regularly checked to confirm the system is functioning within the range of allowable variance, which was used to create the design space (53). Adoption of such systems is predicted to lead to improvements in product quality consistency and manufacturing efficiency, bringing us closer to genuine QbD implementation and the realization of its benefits (54).
13. QTPP Matching with the Finished Product
Once the formulation is optimized in the normal operating space, all the parameters, such as dosage strength, pharmacokinetic properties, content uniformity, assay, and shelf life, will be matched with the predetermined QTPP. Finally, the evaluation test of the final product will be carried out to satisfy the product quality profile, and the product that passes the test will be ready for marketing (42). This also helps serve as a guide for product development when the initial QTPP parameters match the limits of the final parameters of the drug product to ensure the safety, efficacy, and quality of the product (55).
14. Conclusions
Transdermal patch systems are one of the complex dosage forms that have multiple impacts on safety and efficacy. It is necessary to achieve the consistency of the product by understanding the manufacturing process and the CQAs that influence the final performance of the TPS. The aim of this comprehensive review was to provide an excellent assessment of the QbD concept in product development, providing quality medicines to patients, production improvements to manufacturers with significantly reduced batch failures, and formulating the robust quality of products. Understanding and implementing QbD elements, such as QTPP, CQAs, design space, and control strategy, will enhance and modernize the pharmaceutical industry by establishing priorities in the areas of technology transfer, quality, production, and regulatory compliance. Therefore, QbD is considered a potential scientific tool in quality assurance in the pharmaceutical industry. In the end, the ultimate goal is achieved by utilizing the scientific principles, and it proves that by working together, challenges faced by industries, academic, and regulatory elements were overcome and promoted the significant benefits of using QbD in TPS.