1. Background
Acute pulmonary embolism (PE) is a serious clinical manifestation of venous thromboembolism and a common cause of sudden death. In the United States, PE affects approximately 500,000 patients annually, resulting in 200,000 to 300,000 deaths each year (1). Pulmonary embolism ranks as the third most common cardiovascular condition after heart attack and stroke, with an annual prevalence of 1 to 2 per 1,000 individuals (2, 3). Risk factors for PE include obesity, inactivity, smoking, cancer, surgery, trauma, pregnancy, oral contraceptive use, hormone therapy, and a family history of hereditary coagulation disorders. However, 30% of patients with PE have no identifiable risk factors.
Pulmonary embolism is classified into two main categories: Massive (high-risk) and submassive (moderate-risk). Massive PE is characterized by hemodynamic instability, which includes symptoms such as hypotension, absent pulse, or loss of consciousness with signs of shock. Submassive PE is an acute form without systemic hypotension but still involves right ventricular (RV) dysfunction and myocardial necrosis (4, 5).
The primary goals in managing acute PE are preventing embolization and its spread. Thrombolysis is mainly indicated for patients with massive PE who exhibit hemodynamic instability. Additional indications include RV dysfunction, recurrent embolism, or prevention of pulmonary hypertension. Treatment methods are chosen based on the severity of the PE and include anticoagulants, catheter-based thrombolysis, embolectomy, inferior vena cava filters, and systemic thrombolysis with hemodynamic support (6, 7). Thrombolytics increase cardiac output by dissolving the clot and reducing pulmonary artery resistance and pressure, showing superiority over injectable anticoagulants, such as heparin, in the treatment of submassive PE (8-10). Known thrombolytic agents include streptokinase, urokinase, alteplase, and tenecteplase (11-13).
Despite numerous studies on the efficacy of thrombolytics in treating acute PE, limited research has focused on the effectiveness of reteplase for high- and moderate-risk (massive and submassive) PE, emphasizing the need for further investigation.
2. Objectives
This study was conducted to evaluate the use of reteplase for thrombolysis in patients with acute PE requiring thrombolysis in the cardiac wards of Valiasr and Razi hospitals in Birjand, Iran, over a five-year period.
3. Methods
This observational descriptive-analytical study examined patients with acute massive and submassive PE who were hospitalized in the cardiac wards of Valiasr and Razi hospitals in Birjand over a five-year period, from early 2016 to late 2020, and who received thrombolysis with reteplase. The study was conducted after approval from the ethics committee of Birjand University of Medical Sciences, including obtaining an ethics code and a letter of introduction. All patients diagnosed with massive or submassive acute PE and treated with reteplase during the specified period were selected from the target population using the census method and included in the study. Eligible patients were those with massive or submassive pulmonary thromboembolism who had no contraindications for receiving thrombolysis. Reteplase was administered according to the acute STEMI protocol, with two separate bolus injections of 10 IU, given 30 minutes apart. Absolute contraindications included any previous intracranial hemorrhage, known structural cerebral vascular lesions (e.g., arteriovenous malformation), known malignant intracranial neoplasm (primary or metastatic), ischemic stroke within the past 3 months (except for acute ischemic stroke within 4.5 hours), suspected aortic dissection, active bleeding or bleeding diathesis (excluding menses), significant closed-head or facial trauma within the past 3 months, intracranial or intraspinal surgery within the past 2 months, and severe uncontrolled hypertension unresponsive to emergency therapy. Relative contraindications included a history of chronic, severe, poorly controlled hypertension; significant hypertension at initial evaluation (SBP > 180 mmHg or DBP > 110 mmHg); a history of ischemic stroke more than 3 months prior; dementia or known intracranial pathology not covered in absolute contraindications; traumatic or prolonged cardiopulmonary resuscitation (> 10 minutes); major surgery within the past 3 weeks; recent internal bleeding (within 2 - 4 weeks); noncompressible vascular punctures; pregnancy; active peptic ulcer; and ongoing oral anticoagulant therapy.
A total of 28 patients were included in the study. Data were collected from patient records, and for multi-year follow-up on thrombolysis complications, available patients were contacted directly. The data collected included demographic information (age, sex, and marital status), social history, medical history, and history of deep vein thrombosis (DVT). Additionally, parameters such as the time from symptom onset to receiving reteplase, vital signs before and during the 48 hours following reteplase administration—including heart rate (HR), respiratory rate (RR), blood pressure (BP), and significant improvement in hypoxemia (SPO2) (oxygen saturation)—were recorded. Other factors included ECG changes at the time of admission, cardiac troponin levels, Doppler ultrasound reports of lower limb veins (to check for concurrent DVT), and pulmonary vessel CT angiography to determine clot location. Echocardiographic findings, such as RV diameter, pulmonary artery systolic pressure, and tricuspid annular plane systolic excursion (TAPSE), indicating RV systolic function before and after treatment, were also collected. Complications following drug administration, including mortality, thromboembolism recurrence, and bleeding events (major, minor, and stroke), were recorded.
Data analysis was conducted using appropriate descriptive and analytical statistics, based on data normality, with SPSS version 22. Paired t-tests were used to assess changes in parameters within the massive and submassive groups before and after thrombolysis. The independent t-test was employed to compare results between the two groups, or the Mann-Whitney test was used in cases of non-normal distribution. For non-parametric analyses, the Wilcoxon and McNemar tests were used in place of paired t-tests where applicable.
4. Results
A total of 28 patients (14 women and 14 men) participated in this study, with an average age of 54 ± 12 years. The average age for female patients was 55 ± 13 years, and for male patients, it was 52 ± 12 years. All patients were married. Among them, 35% had hypertension, 14% had diabetes, and 17% had dyslipidemia. Additional demographic information and details regarding comorbidities are provided in Tables 1, 2 and 3.
| Variables | No. (%) |
|---|---|
| Diabetes melitus | 4 (14.3) |
| Hypertension | 10 (35.7) |
| Dyslipidemia | 5 (17.9) |
| Ischemic heart diseases | 8 (28.6) |
| Congestive heart failure | 1 (3.6) |
| Stroke | 3 (10.7) |
| History of acute infection | 4 (14.3) |
| Active cancer | 2 (7.1) |
| Treated cancer | 0 (0) |
| Plaster cramps in the last month | 2 (7.1) |
| History of surgery in the last 4 weeks | 8 (28.6) |
| History of hospitalization up to three days | 2 (7.1) |
| History of hospitalization for more than three days | 12 (42.9) |
| Hypercoagulation | 0 (0) |
| Under anticoagulant treatment beforehand | 2 (7.1) |
| Concomitant DVT | 12 (42.9) |
| History of previous venous thromboembolism in the patient | 5 (17.9) |
| Family history of venous thromboembolism | 3 (10.7) |
| Hormone replacement therapy, pregnancy, postpartum period | 0 (0) |
| Family history of venous thromboembolism | 2 (7.1) |
Abbreviation: DVT, deep vein thrombosis.
| Variables | No. (%) |
|---|---|
| Smoking | 4 (14.3) |
| Air travel | 0 (0) |
| Long-term travel | 0 (0) |
| Variables | Mean ± SD | First Quarter | Median | Third Quarter | P-Value |
|---|---|---|---|---|---|
| SPO2 | < 0.001 | ||||
| When hospitalizing | 83 ± 5 | 79 | 85 | 88 | |
| After receiving reteplase | 93 ± 3 | 90 | 94 | 96 | |
| Breathing rate | < 0.001 | ||||
| When hospitalizing | 27 ± 5 | 24 | 26 | 30 | |
| After receiving reteplase | 19 ± 3 | 17 | 18 | 21 | |
| Heart beat | < 0.001 | ||||
| When hospitalizing | 115 ± 18 | 100 | 120 | 129 | |
| After receiving reteplase | 88 ± 10 | 80 | 86 | 97 | |
| Cytolic BP | < 0.001 | ||||
| When hospitalizing | 93 ± 19 | 80 | 86 | 107 | |
| After receiving reteplase | 119 ± 10 | 110 | 120 | 130 | |
| Diastolic BP | < 0.001 | ||||
| When hospitalizing | 59 ± 14 | 50 | 59 | 71 | |
| After receiving reteplase | 77 ± 7 | 71 | 80 | 81 |
Abbreviations: BP, blood pressure; SPO2, significant impact on improving hypoxemia.
Our results indicated that 48 hours after reteplase administration—given as two separate bolus injections of 10 IU, 30 minutes apart, similar to the acute STEMI protocol—there was a SPO2 and vital signs, including BP, RR, and HR (P < 0.001). The patients’ vital signs (BP, HR, RR, and SPO2) before hospitalization and after receiving reteplase are shown in Table 3.
Upon admission, 64% (18 patients) presented with hypotension, but none exhibited hypotension 48 hours after reteplase administration. The hypotension status of patients before and after receiving reteplase is detailed in Table 4.
| Variables | No. (%) | P-Value |
|---|---|---|
| Hypotension before receiving reteplase | 18 (64.3) | < 0.001 |
| Hypotension after receiving reteplase | 0 (0) |
The results of McNemar’s test demonstrated that reteplase significantly improved dyspnea in patients with acute massive and submassive PE (P < 0.001). Initially, 42% (12 patients) reported chest pain, yet no chest pain was reported 48 hours after receiving reteplase. Additionally, only one patient (3%) reported hemoptysis upon admission, and this symptom was absent 48 hours after treatment. The results showed that reteplase, administered following the acute STEMI protocol, had a significant positive effect on chest pain and syncope (P < 0.001 and P = 0.031, respectively). However, it had no significant effect on hemoptysis or cough (P > 0.05). The clinical symptoms of patients at admission and 48 hours post-reteplase administration are provided in Table 5.
| Variables | Before Receiving Reteplase | After Receiving Reteplase | P-Value |
|---|---|---|---|
| Shortness of breath | 27 (96.4) | 4 (15.4) | < 0.001 |
| Hemoptysis | 1 (3.6) | 0 (0) | > 0.05 |
| Chest pain | 12 (42.9) | 0 (0) | < 0.001 |
| Cough | 6 (21.4) | 0 (0) | 0.063 |
| Syncope | 8 (28.6) | 0 (0) | 0.031 |
a Values are expressed as No. (%).
The results also indicated that reteplase, following the acute STEMI protocol, had a significant effect on RV diameter 48 hours post-treatment, as compared to the diameter at admission (P = 0.045). Similarly, a significant improvement in RV function (TAPSE) was observed 48 hours after reteplase administration (Table 6) (P < 0.01).
| Variables | Mean ± SD | P-Value |
|---|---|---|
| RV diameter | 0.045 | |
| Before receiving reteplase | 36 ± 16 | |
| After receiving reteplase | 30 ± 10 | |
| RV function | < 0.01 | |
| Before receiving reteplase | 12 ± 4.07 | |
| TAPSE | ||
| After receiving reteplase | 17.08 ± 5 | |
| PRP | < 0.001 | |
| Before receiving reteplase | 57 ± 16 | |
| After receiving reteplase | 34 ± 12 |
Abbreviations: RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion.
Reteplase also had a significant effect on pulmonary arterial systolic pressure (PASP) observed in echocardiography at least 48 hours after administration, compared to PASP immediately upon hospitalization (P < 0.001). The changes in RV parameters (RV diameter, pulmonary artery systolic pressure, and TAPSE) before and after reteplase administration are displayed in Table 7.
| Variables | Mean± SD | 1st Quarter | Median | 3rd Quarter | P-Value |
|---|---|---|---|---|---|
| Age | < 0.001 | ||||
| Responding to treatment | 52 ± 12.02 | 42 | 55 | 60 | |
| Not responding to treatment | 59 ± 15 | 41 | 63 | 71 |
Doppler ultrasound of the lower limb veins showed no vein involvement in 55% (n = 15) of patients, while DVT was observed in 44% (n = 12).
The results of the Mann-Whitney test indicated a significant relationship between response rate to reteplase treatment and patient age in cases of massive and submassive acute PE (P < 0.001). Age status and response to reteplase treatment are presented in Table 7.
The Phi correlation coefficient results revealed no statistically significant relationship between treatment response rate with reteplase and patient gender (P > 0.05), with 11 men and 11 women responding positively to the treatment, while 3 men and 3 women did not respond.
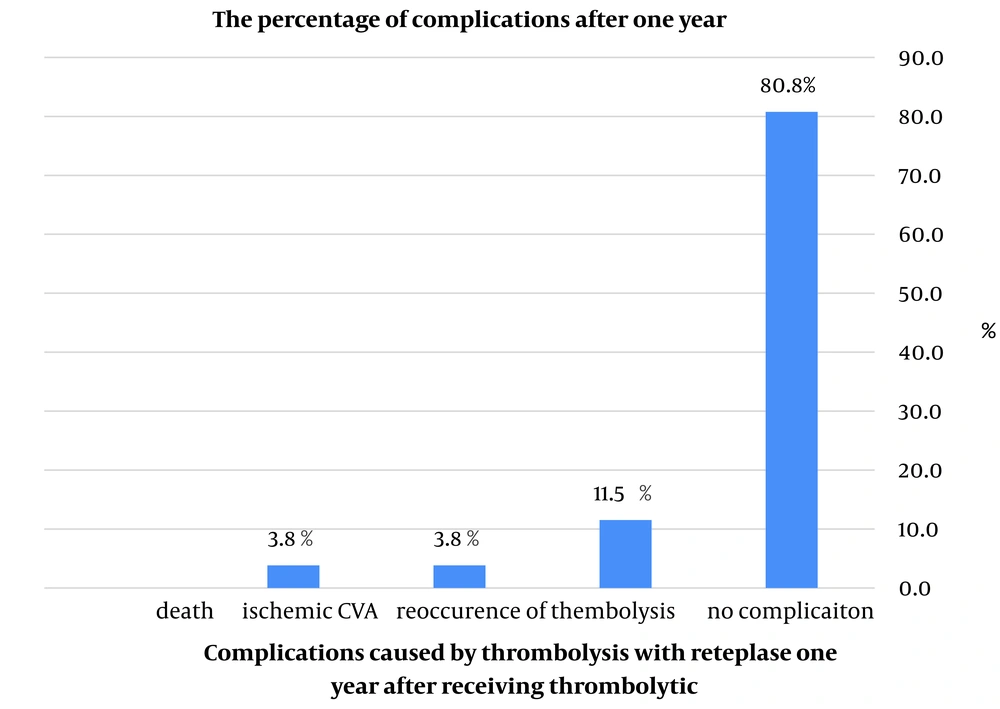
Reteplase had no side effects in 92.8% of patients (26 individuals) during hospitalization. Among the 26 discharged patients, 21 (80.8%) did not report any complications post-discharge, while 5 (19.2%) reported complications. Thromboembolism recurrence was noted in only 3 patients (11.5%), and ischemic cerebrovascular accident (CVA) occurred in only one patient (3.8%). The overall mortality rate in our study was 10.7% (3 patients), with two deaths occurring during hospitalization and one death within a year after discharge. Additional patient details, including average age, gender, and underlying conditions, are provided in Table 8. The percentage of complications from thrombolysis with reteplase in acute massive and submassive PE patients one year after administration is shown in Figure 1.
| Gender | Age | Background diseases and risk factors | Complication |
|---|---|---|---|
| Man | 80 | History of surgery in the last 4 weeks, history of hospitalization for more than three days | Death in hospital |
| Female | 42 | Hypertension (it should be noted that the patient was admitted to the emergency ward with cardio-respiratory arrest, and according to the evidence of severe RV failure in the echocardiography, with the possibility of massive PTE, and was treated with reteplase) | Death in hospital |
| Female | 69 | High BP, ischemic heart diseases, history of acute infection | He died 11 months later |
| Female | 55 | High BP, history of acute infection, cast in the last one month, history of surgery in the last 4 weeks, history of hospitalization for more than three days, simultaneous DVT, venous thromboembolism in the family | Recurrence of thromboembolic events |
| Man | 41 | History of hospitalization up to three days, simultaneous DVT | Recurrence of thromboembolic events |
| Female | 48 | Dyslipidemia, active cancer, plaster cast in the last month, history of hospitalization for more than three days, family history of venous thromboembolism | Ischemic CVA |
| Man | 60 | Ischemic heart diseases, stroke, smoking | Recurrence of thromboembolic events |
Abbreviations: CVA, cerebrovascular accident; BP, blood pressure; RV, right ventricular.
5. Discussion
Thrombolysis is an established treatment for high-risk (severe) acute PE with hemodynamic instability (14, 15). This approach effectively dissolves the thromboembolic blockage, rapidly lowering pulmonary artery pressure and resistance by increasing cardiac output (11). Thrombolysis has also shown superiority over anticoagulant therapy in treating moderate-risk PE (16). Proven thrombolytic agents include streptokinase, urokinase, alteplase, and tenecteplase. Reteplase, a third-generation fibrin-specific recombinant tissue plasminogen activator, lacks the epidermal growth factor and Kringle domain 1 (17). While the efficacy of reteplase in acute myocardial infarction is well-documented, only limited case reports and series have addressed its use in high- and moderate-risk PE. Consequently, this study aims to evaluate the effectiveness of thrombolysis with reteplase on vital signs, RV parameters, and clinical symptoms in patients with acute massive and submassive PE requiring thrombolysis, conducted in the cardiac ward of Valiasr and Razi hospitals in Birjand from early 2016 to late 2020.
In this study, a total of 28 patients, comprising 14 men and 14 women with an average age of 54 ± 12, were included. Shortness of breath was reported by 96.4% of patients. Previous studies have also identified dyspnea as the most common symptom, aligning with our findings.
Hypotension was initially reported in 64.3% of patients (n = 18), but 48 hours after receiving reteplase, none of the patients exhibited hypotension. Reteplase SPO2 and key vital signs, including HR, RR, and BP upon admission (18, 19). It also had a notable positive effect on dyspnea in patients with acute massive and submassive PE (P < 0.001). Initially, 42.9% (12 patients) reported chest pain, but none reported it 48 hours after receiving reteplase. Only one patient (3.6%) reported hemoptysis at admission, and this symptom was absent 48 hours post-treatment.
In a retrospective study of 20 patients with massive PE treated with reteplase, findings demonstrated improved RV function and reduced systolic pulmonary artery pressure (20). In our study, reteplase also had a significant effect on RV diameter and function (TAPSE) observed via echocardiography 48 hours after administration compared to measurements taken upon hospitalization. Additionally, reteplase significantly reduced pulmonary artery systolic pressure (PASP), decreasing from 57 ± 16 mmHg to 34 ± 12 mmHg, according to echocardiography results.
Reteplase exhibited an excellent safety profile, with no side effects reported in 92.8% (26 patients) during hospitalization. Of the 26 discharged patients, 21 (80.8%) reported no complications after discharge, while only 5 (19.2%) noted minor complications. Thromboembolism recurrence was observed in just 3 (11.5%) patients, and one patient (3.8%) experienced an ischemic CVA.
In a study by Tebbe et al., comparing reteplase and alteplase, no significant difference in safety outcomes was observed, and no stroke or intracranial hemorrhage occurred in patients with severe PE. This study also demonstrated that reteplase, administered in a double standard bolus of 10 + 10 U, is effective for treating severe PE (21). In another study by Zhang et al., only one death was reported, with significant improvement observed in 11 cases, and the effective rate of reteplase treatment was 93.8%. In Zhang’s study, HR and RR remained similar before and after thrombolysis. In contrast, our study reported three deaths, with decreases in HR and RR following reteplase treatment. Furthermore, Zhang's study reported no life-threatening bleeding in the reteplase group, a finding consistent with our results (22).
A review of randomized trials with other thrombolytic agents found mortality rates of 9.4% for high-risk PE and 2.2% for intermediate-risk PE following reteplase administration (7). In our study, the overall mortality rate was 10.7% (3 patients), including two who died during hospitalization and one within a year post-discharge. Notably, one of the hospitalized patients had experienced cardiopulmonary arrest and showed evidence of severe RV failure, indicative of massive PE. Zhang et al. also reported an excellent safety profile for reteplase, with no major bleeding, aligning with our findings, where most patients reported no complications after discharge (22).
In a case report, Çoner et al. demonstrated the successful treatment of massive PE with reteplase administered as two separate bolus injections of 10 IU, spaced 30 minutes apart. After the administration of the first bolus, return of spontaneous circulation (ROSC) was achieved within 6 minutes. Duplex ultrasound of the lower limb detected acute thrombosis in the deep right femoral vein. The dilation and dysfunction of the right ventricle decreased, and mean pulmonary arterial pressure fell below 15 mmHg (23). In another case report by Theron and Laidlow, reteplase (10 U followed by another 10 U after 30 minutes) significantly improved the patient's cardiovascular status (24). Similarly, in a study by Zhang et al., conducted on an animal model (dog) of pulmonary thromboembolism, a single bolus injection of reteplase (6.0 mg/kg) over 2 minutes led to a normalization of pulmonary systolic, diastolic, and mean arterial pressure (25).
Almost all studies, including the present one, have shown rapid and significant improvement in hemodynamic and echocardiographic parameters. Overall, the results of our study, along with findings from other studies, indicate that reteplase effectively reduces symptoms and improves clinical factors associated with pulmonary thromboembolism, with fewer complications and lower mortality compared to other thrombolytic and anticoagulant drugs. Evidence from this retrospective study, along with previous case reports, suggests that reteplase is highly effective for PE and exhibits an excellent safety profile. This study adds positive evidence supporting the increased use of reteplase for thrombolysis, positioning it as a suitable alternative to first-generation thrombolytics in PE. For more definitive conclusions, future research in the form of large prospective and randomized studies is needed.
Our study has several limitations, the most notable being the lack of a comparison arm. Additionally, as a retrospective study, the number of patients receiving reteplase for thrombolysis was relatively small.
5.1. Conclusions
Pulmonary embolism is a life-threatening condition that requires prompt systemic thrombolysis. In our study, reteplase demonstrated high efficacy, leading to rapid clinical improvement. Additionally, it was used without significantly increasing the risk of bleeding or mortality. Although this study was limited by its retrospective design, reteplase appears to be a promising option for treating PE. However, further large-scale prospective studies are needed to confirm these findings.