1. Background
Type II diabetes, a chronic metabolic disorder, is characterized by inadequate insulin levels and increased insulin resistance, leading to hyperglycemia (1). Insulin regulates glycogen production and decreases the expression of genes involved in gluconeogenesis. Disruptions in these processes can result in insulin resistance, a condition where peripheral tissues become less responsive to insulin signals. Although the pancreas produces insulin, tissues fail to absorb it efficiently from the bloodstream. This leads to elevated blood sugar levels, which can cause hyperinsulinemia, hyperglycemia, and eventually diabetes (2). This condition is closely associated with obesity and the accumulation of excess fat in the liver (1). Hyperglycemia results in unbalanced oxidative stress, especially in the liver, where free radicals are produced at high levels, challenging the body's antioxidant system (3). In this context, damage should be addressed, and apoptosis, as a protective mechanism, becomes more pronounced (4). The critical role of apoptosis in the progression of diabetic complications is well established, leading to an increasing demand for agents that can modulate this process. Generally, antioxidant agents are excellent candidates to compensate for the deficiency caused by oxidants, among which plant secondary metabolites are considered the best resources and significant hypoglycemic agents introduced in the past decades (5). Due to their minor side effects, affordability, and availability, pharmaceutical plants are gaining increasing attention (6). Peganum harmala, a well-known member of the family Zygophyllaceae, is a rich source of alkaloids and flavonoids and has been widely used traditionally in Iran (7). Seeds of P. harmala are well-known sources of alkaloids such as harmine, harmaline, and harmalol, which exhibit substantial anti-inflammatory and antioxidant effects (8). Previous studies on induced diabetic rats support the idea that alkaloids in the hydroalcoholic extracts of P. harmala effectively reduce blood glucose levels and improve liver function (9). Moreover, the positive effects of P. harmala extracts on lipid profiles in diabetic complications have been reported (10).
2. Objectives
In this study, we aimed to investigate the apoptosis process in the liver tissue of induced diabetic Wistar male rats in the presence of methanolic extracts of P. harmala prepared from its seeds and leaves via flow cytometry. Additionally, we evaluated biochemical and histopathological parameters in induced diabetic rats receiving defined doses of methanolic extracts of P. harmala. To gain better insight, pure harmine was utilized in all experiments.
3. Methods
3.1. Chemicals
Lyophilized harmine (98%), streptozotocin (STZ), and enzymes (trypsin and DNase I) were purchased from Sigma-Aldrich (USA). Other chemicals, such as methanol, were procured from reputable brands, including Merck (Germany) and Sigma-Aldrich (USA). Analytic biochemical kits were obtained from Pars Azmoon Co. (Iran), and the annexin-PI kit was purchased from BioLegend (USA). ELISA tests were read using a BioTek (USA) apparatus.
3.2. Plant Material
Peganum harmala was collected from an area 5 km outside Zahedan city. The plant was confirmed at the Central Herbarium of Iran (TARI) with the number 107208. The leaves and seeds were separated and dried at room temperature. Extraction was performed using a Soxhlet extractor (middle section) with 500 mL of 80% methanol as a solvent for 3 to 4 hours. After collecting the extracts, they were stored at 4°C. Phytochemical analysis was conducted, and metabolites were characterized by high-performance liquid chromatography (HPLC) using an analytical column (C18) and a mobile phase consisting of isopropyl alcohol:acetonitrile:water:formic acid (100:100:300:3) at a flow rate of 1 mL/min.
3.3. Animals’ Experiments
Sixty-four male Wistar rats (250 - 300 g) were purchased from the Pasteur Institute of Iran. They were maintained under laboratory conditions with a 12-hour light/dark cycle and had access to standard food and water. After a one-week adaptation period, the rats were randomly divided into eight groups of eight individuals, with each group housed in two cages equally. The animal protocol was assessed and approved by the ethics committee of the Islamic Azad University, Science, and Research Branch, under the number IR.IAU.SRB.REC.1396.37. The grouping was as follows:
(1) Diabetic control group (DC): Diabetic without any treatment.
(2) Healthy control group (C): Healthy without any treatment.
(3) Experimental group 1 (H): Healthy treated with harmine.
(4) Experimental group 2 (S): Healthy treated with seed extract.
(5) Experimental group 3 (L): Healthy treated with leaf extract.
(6) Diabetic group 1 (DH): Diabetic treated with harmine.
(7) Diabetic group 2 (DS): Diabetic treated with seed extract.
(8) Diabetic group 3 (DL): Diabetic treated with leaf extract.
3.4. Diabetes Induction and Treatments
Streptozotocin at a dosage of 70 mg/kg, dissolved in distilled water, was injected intraperitoneally into the rats. Forty-eight hours to ten days after receiving STZ, the plasma glucose level for each rat was determined using a glucometer, with levels higher than 200 mg/dL indicating diabetes. Additionally, polydipsia and polyuria were checked as diabetic symptoms 5 to 7 days post-injection. The treatment groups received 150 mg/kg of either leaf or seed extract, or a dose of 6.5 mg/kg of harmine daily for four weeks via gavage (11). Harmine was dissolved in saline (0.9% NaCl) prior to gavage administration. Upon completion of the treatment period, rats were anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg). The rats were sacrificed in accordance with ethical guidelines, ensuring minimal suffering and distress. All procedures were approved by the institutional ethics committee. An open surgery was then performed to obtain the desired tissue portions and blood samples. Blood samples were collected directly from the heart using a 5 mL syringe after opening the chest surgically.
3.5. Biochemical Tests
Blood samples were transferred to 10 mL tubes and allowed to coagulate at room temperature for 30 minutes. They were then centrifuged for 10 minutes at 5000 RPM. Serum was separated from the blood cells and centrifuged again for 10 minutes at 5000 RPM. The final supernatant was isolated and stored at 2°C to 8°C. Biochemical tests, including measurements of cholesterol, triglycerides, alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase plasma levels, were performed using an autoanalyzer apparatus (Alpha-Classic AT Plus, Iran). The ELISA assay was conducted according to the manufacturer's instructions (11).
3.6. Apoptotic Experiments
To evaluate the apoptosis process, desired parts of the liver tissue were isolated and prepared for single-cell production as described elsewhere. The resulting single cells were then assessed using a flow cytometer device (BD FACSCalibur, USA). The preparation for flow cytometry is reviewed elsewhere. The data obtained from flow cytometry were analyzed using FlowJo software (versions 10.5.3/10.0.7 R2) (11).
3.7. Histopathological Evaluations
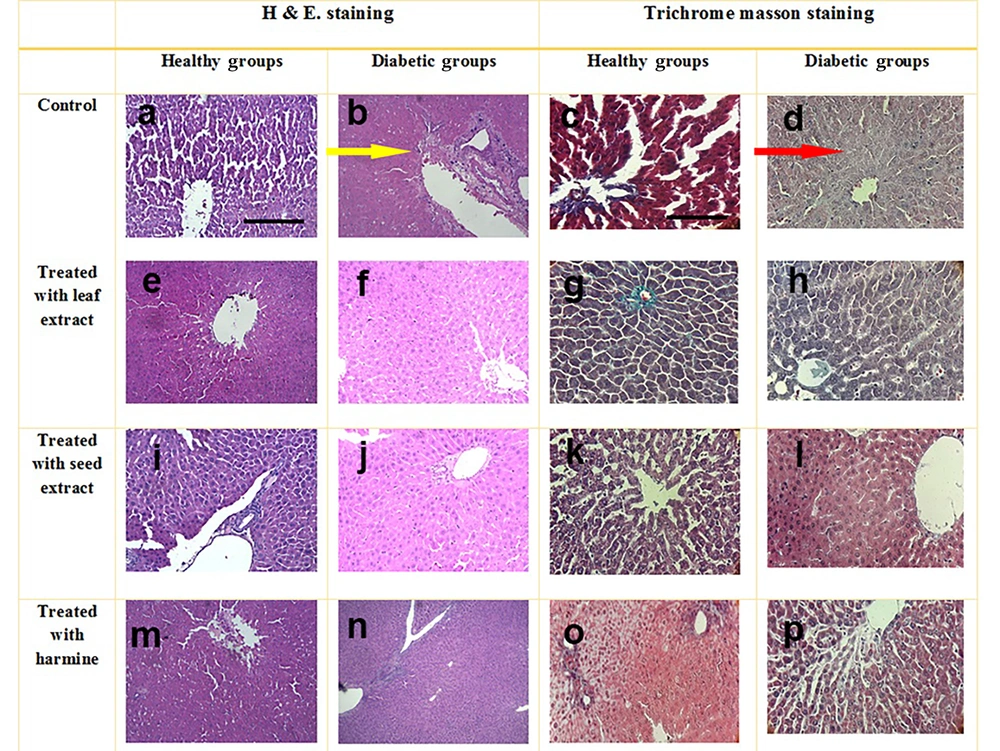
Appropriate portions of the liver tissue were removed and fixed in 10% formalin. The preparation of liver tissue samples for histopathological evaluations is described elsewhere. Desired sections, with a thickness of 5 microns, were obtained using a microtome instrument (YD 1508A). Subsequently, the prepared tissue samples were evaluated using hematoxylin and eosin (H&E) staining as well as Masson's trichrome staining. Finally, these slides were observed under a microscope (Olympus BX51, Japan) (11).
3.8. Statistical Analysis
Statistical analyses were performed using ANOVA and the Tukey test, with statistical significance defined as P < 0.05. All data were analyzed using SPSS statistical software, version 21.
4. Results
4.1. Phytochemical Analysis
The results revealed that β-carboline and quinazoline alkaloids are the most commonly found compounds in P. harmala. The total alkaloid content was found to be 7% in the seeds, with higher concentrations of harmaline and harmine. These results are detailed in Table 1.
| Extract Type | Seed | Leaf |
|---|---|---|
| Extraction percentage | 29.7 | 31.7 |
| Alkaloid (%) | ||
| Harmalol | 0.12 | 0.026 |
| Harmol | 0.02 | - |
| Harmane | 0.029 | 0.011 |
| Harmaline | 3.8 | 0.05 |
| Harmine | 2.93 | 0.055 |
| Total flavonoid contents (mg QE/mg extract) | 16.63 | 25.84 |
| Total phenolic contents (mg GAE/mg extract) at 200 mg/mL | 30.46 | 32.84 |
| Anti-oxidant | ||
| Scav (%) | 68.9 | 34.7 |
| IC50 | 122.1 | > 200 |
Phytochemical Analysis of Methanolic Extracts of Peganum harmala by High-Performance Liquid Chromatography and DPPH Test
4.2. Biochemical Tests
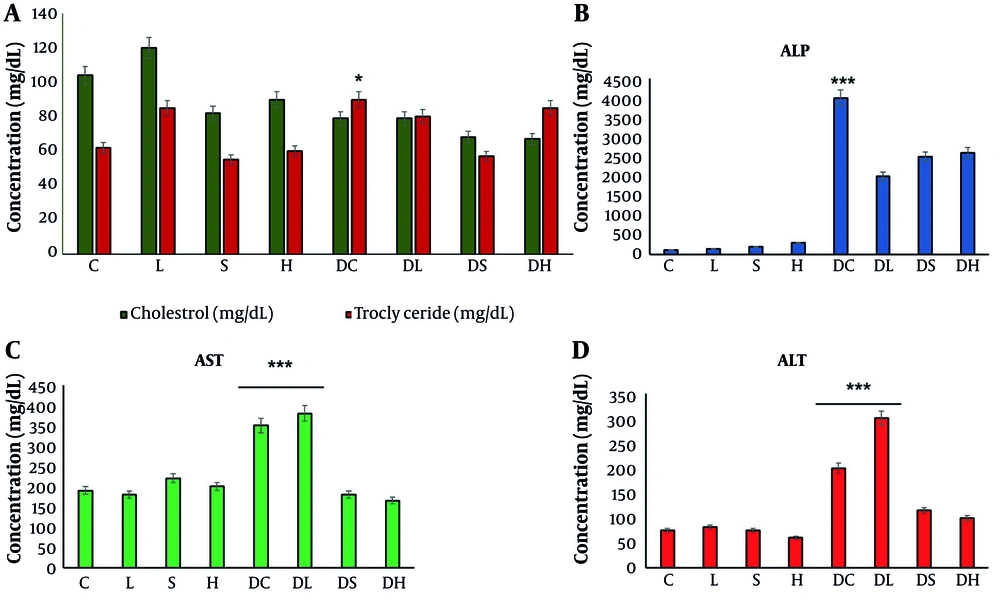
The study conducted various biochemical assays on plasma samples, which included measuring cholesterol and triglyceride plasma levels, as well as ALP, AST, and alanine aminotransferase plasma levels. The findings are illustrated in Figure 1. As shown in Figure 1A, in the DC, cholesterol levels were significantly increased compared to the healthy control group (C). It was observed that the cholesterol levels in groups treated with seed extract (S and DS) and harmine (H and DH) were lower than those in leaf extract-treated groups (L and DL). Similar to cholesterol, triglycerides were also increased in the DC. In terms of triglyceride plasma level reduction, seed extracts (S and DS) performed better than harmine and leaf extracts (H, DH, L, and DL). The results indicated that ALP was significantly increased in the diabetic groups; however, treatments with the seed and leaf extracts, as well as harmine, resulted in a reduction of ALP among diabetic groups (Figure 1B). According to Figure 1C, AST plasma levels were significantly elevated in the diabetic groups, with a reduction observed only in DS and DH, while AST plasma levels remained unchanged in the DL group compared to the DC group. Evaluating ALT plasma levels revealed similar outcomes to AST's. Meanwhile, the DL group exhibited the highest AST plasma level (Figure 1D).
4.3. Apoptotic Experiments
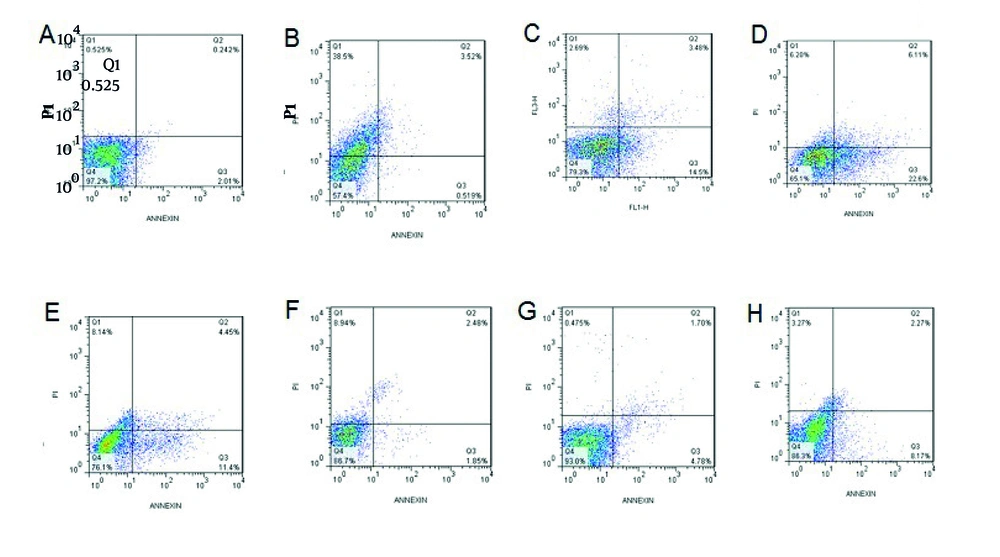
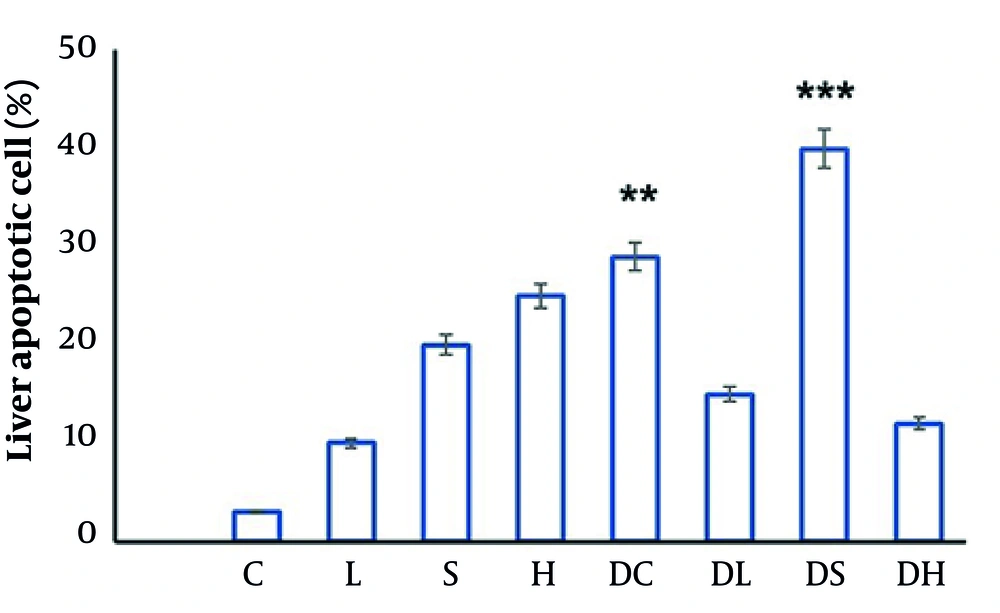
In this section, prepared single cells from the liver tissue were analyzed using the flow cytometry method to evaluate the effect of treatments on liver cell apoptosis. These results are presented in Figure 2. To clarify the results of flow cytometry, the apoptosis rate in each group is summarized in Figure 3. Based on the results presented in Figure 3, apoptosis was significantly increased among the diabetic groups. Among healthy rats, the harmine-treated group (H) showed a higher rate of apoptosis compared to the control group (C). It was also revealed that in the diabetic groups, apoptosis had a higher rate in the DS group compared to the DL group.
4.4. Histopathological Evaluations
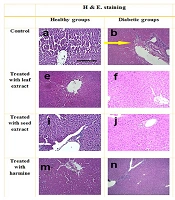
When the slides were prepared, examinations via a microscope were conducted, and staining with H&E and Masson's trichrome was applied to the samples. These results are presented in Figure 4. Histopathological alterations in the liver tissue included fatty degeneration, necrosis, fibrosis, blood vessel congestion, and bleeding. Histopathological evaluations indicated that the liver tissues in healthy rats (C) were completely normal, with intact lobes and no observed tissue damage. In contrast, histologic abnormalities in the liver tissue were observed in the DC group. Meanwhile, the liver tissues of diabetic animals receiving extracts appeared mostly normal, with no major alterations observed. In the DS and DH groups, most hepatocytes showed intact cytoplasm, bright staining, and preserved membranes. In the DL group, although some abnormal cells were observed, the majority of the tissue exhibited normal features. Overall, the seed extract seemed to support the recovery of liver tissue by reducing damage.
Stained samples from the liver tissue with hematoxylin and eosin (H&E) and trichrome masson. Histologic abnormalities in the liver tissue of diabetes group indicated by arrows. b, yellow arrow: Necrosis, alterations in architecture; d, red arrow: Extensive fibrosis; i, reduction in necrosis and inflammation; h, reduced fibrotic areas; p, minimal fibrosis. Scale bar 150 µm, magnification 100X
5. Discussion
In the following study, it was revealed that alkaloids are the major fraction of the compounds occurring in extracts from the seeds of P. harmala, which is consistent with previous studies reporting that alkaloids are primarily accumulated in the roots and seeds of P. harmala, while in the stems and leaves, alkaloids exist in lower concentrations, and flowers lack these compounds (12-14). Thus, the seed extract was enriched with β-carboline (15), while polyphenols and flavonoids are more expected in leaf extracts of P. harmala, leading to a higher IC50 score and fewer antioxidant characteristics (16). Therefore, seeds of P. harmala are the main candidates for pharmaceutical purposes. Moreover, it should be noted that these characteristics make them more cytotoxic.
Furthermore, the results indicated that the seed extract reduced plasma cholesterol and triglyceride levels among all studied groups. The seed extract of P. harmala significantly reduced LDL levels, likely due to the presence of alkaloids such as harmine and harmaline (17). Another study demonstrated that hydroalcoholic extracts of P. harmala decreased both triglyceride and cholesterol plasma levels, increased HDL, and maintained liver enzyme levels (18). Abd El Baky et al. (19) suggested that this reduction in lipid levels may be attributed to the elimination of free radicals due to the antioxidant properties of the seed extract. Although antioxidant enzymes were not directly measured in this study, the potential impact on lipid levels may be linked to the alkaloid content, which has been reported to increase plasma antioxidant capacity and reduce oxidative stress in other studies (20).
Furthermore, biochemical tests in this study revealed that groups treated with P. harmala showed a reduction in liver enzyme plasma levels. However, groups treated with seed extracts and harmine showed that ALT, AST, and ALP plasma levels were significantly reduced. These results are consistent with a previous study, which also reported a significant reduction in these liver enzymes in rats treated with P. harmala extract (21). Therefore, it can be concluded that β-carbolines present in the seeds of P. harmala are particularly effective in reducing plasma levels of liver enzymes.
The impact of STZ on the induction of diabetes in rats has been extensively studied, with evidence suggesting that induced hyperglycemia leads to an increase in the rate of apoptosis, potentially contributing to liver diseases (22). This study aimed to assess the effects of various treatments on apoptosis rates in Wistar male rats. Previous research has indicated that diabetes induction generally results in increased apoptosis in various areas of the body (23). Moreover, it has been reported that hyperglycemia can increase the release of hydroxyl free radicals, ultimately activating caspase 3 through a signaling cascade, leading to apoptosis (24).
The current study found that the leaf extract and harmine significantly reduced apoptosis in diabetic groups. On the other hand, seed extracts showed a more complex effect, with some groups experiencing an increase in apoptosis. This may be attributed to the high alkaloid content of the seeds, which, at certain concentrations, can have cytotoxic effects, potentially leading to increased apoptosis in specific tissues. Previous studies have reported that alkaloids present in P. harmala may promote apoptosis, particularly by inducing DNA damage (25). Nonetheless, despite the observed increase in apoptosis, the seed extracts demonstrated a positive role in liver recovery by reducing inflammation and promoting tissue healing. This suggests that the antioxidant components of the seed extract help neutralize oxidative stress.
According to the obtained data, leaf extracts were found to effectively reduce apoptosis, suggesting that the antioxidant properties of plant compounds may have an inhibitory effect on apoptosis (26). Leaf extracts reduced apoptosis due to their antioxidant properties, resulting from their higher concentration of flavonoids and lower cytotoxicity. This finding highlights the dual nature of plant compounds, where some may act as antioxidants, while others may exhibit cytotoxic effects depending on dosage and exposure.
The histopathological evaluations conducted in the present study revealed that the liver exhibited normal morphology in the healthy control groups, while the DC and the methanol-treated group showed signs of inflammation and necrosis, indicating liver damage. However, the treated groups displayed varying degrees of liver tissue healing and recovery, with the seed extracts demonstrating greater efficacy in promoting healing. Consistent with these findings, a previous study reported that diabetic rats administered P. harmala seed oil did not exhibit any tissue alterations and experienced complete recovery of liver cells. The authors proposed that orally administered P. harmala seed oil possesses anti-tumor, antioxidant, and hypoglycemic properties, which contribute to the healing of liver damage caused by diabetes complications (19). Therefore, the present results suggest that the restoration of liver tissue at the cellular level is associated with the antioxidant effects of seed extracts that eliminate free radicals.
The findings of the present study highlight the promising effects of P. harmala seed extracts, particularly the β-carbolines such as harmine, in alleviating complications associated with diabetes. The extracts demonstrated the ability to reduce blood lipid levels, lower plasma liver enzyme levels, and promote the recovery of liver tissue damage caused by diabetes. These effects are likely due to the potent antioxidant properties of P. harmala seed extracts. Although the current study focused on these metabolic and hepatic parameters, further research with regular monitoring of blood glucose levels would provide deeper insights into the hypoglycemic potential of P. harmala. Moreover, additional studies related to other underlying mechanisms such as inflammation and oxidative stress are needed.