1. Background
The cornu Ammonis 1 (CA1) region of the hippocampus plays a crucial role in learning and memory (1). Neurotoxic lesions in this area have been shown to cause anterograde memory impairment. Administering a neurotoxic dose of N-methyl-D-aspartate (NMDA) into the brain overactivates NMDA receptors, leading to excitotoxic neuronal damage (2), which may contribute to memory deficits (3).
Vitamin K (Vit K) is a fat-soluble vitamin, with growing evidence indicating that phylloquinone, likely converted to menaquinone-4 (MK-4) in tissues, and MK-4 itself play roles in brain function (4, 5). Menaquinone-4, the primary form of Vit K in the brain, is involved in synthesizing sphingolipids like sphingomyelin and ceramide, which are essential components of myelin and neuronal cell membranes (6, 7). Low dietary intake of Vit K has been associated with reduced spatial learning ability in older age, possibly due to increased ceramide concentrations in the hippocampus (8). In one study, rats on a low-Vit K diet showed disruptions in sulfatide metabolism and myelin structure (9). Additionally, MK-4 has antioxidant and anti-inflammatory properties, with evidence suggesting that Vit K may help protect neurons from oxidative damage (10).
Previous studies have primarily examined NMDA-induced neurotoxicity in vitro. However, there is limited research on the impact of NMDA injection into the CA1 region on subsequent memory deficits in vivo. Most research on Vit K has focused on its relationship with cognitive function, particularly its potential protective effects against age-related cognitive decline. In this study, we aimed to assess the therapeutic effects of Vit K following CA1 hippocampal damage, rather than its preventive function.
2. Objectives
The objective of this study was to investigate the effect of NMDA-induced memory impairment using passive avoidance and open-field tests, both with and without Vit K, in male rats.
3. Methods
3.1. Animals
Forty male Wistar rats (initial weight ranging from 200 to 220 gr) were used. In this study, two rats per cage were housed under standard conditions: A 12-hour light/12-hour dark cycle at a temperature of 22 ± 2ºC. All animals had free access to water and food. Experiments were conducted from 8:00 to 12:00 a.m. The procedures were performed in accordance with the Declaration of Helsinki and the guidelines of the Guilan University of Medical Sciences Ethics Committee for the use of experimental animals, under the code IR.GUMS.REC.1394.279.
3.2. Surgery
The animals were anesthetized using a mixture of ketamine and xylazine (90 mg/kg and 10 mg/kg, respectively, administered at 0.1 mL/100 g intraperitoneally). According to the coordinates provided in Paxinos and Watson’s Atlas (11) of the rat brain, three coordinates—anterior-posterior (AP), medial-lateral (ML), and dorsal-ventral (DV)—were used to locate the CA1 region. Two independent 22-gauge guide cannulas were bilaterally implanted over the CA1 region (coordinates: AP from bregma: -3.84 mm; ML: ± 2.2 mm; DV: 2.6 mm). The cannulas were permanently secured to the skull using two screws and dental acrylic. At the conclusion of behavioral testing, methylene blue was injected into the brain using a Hamilton syringe to confirm injection placement. The rats were then anesthetized with chloroform and decapitated after 30 minutes. Brains were extracted, fixed in 10% formalin, and subsequently dissected for histological verification of the injection site.
3.3. Study Design and Drug Injection
Animals were randomly divided into five groups (eight animals in each) as follows: Sham; group NS, which received normal saline; group N, which received NMDA; group NK, which received NMDA + Vit K (phytonadione, a synthetic form of phylloquinone); and group NC, which received NMDA + Vit K solvent (serum dextrose + Cremophor E1, polyethoxylated castor oil, Kollipor EL).
N-methyl-D-aspatate (Sigma-Aldrich, USA) administration into the CA1 area was performed using a stereotaxic technique. After a one-week recovery period, NMDA was injected through the cannula. Injection needles (30 gauge) were connected by polyethylene tubing to a 1 µL Hamilton microsyringe. The injection needle was inserted 1 mm beyond the end of the guide cannula. A single dose of NMDA, dissolved in normal saline (20 µg/µL, 0.2 µL) (12), was injected into each lateral side of the CA1 region over a period of 1 - 2 minutes. To ensure complete diffusion of the solution from the needle tip, the needle was kept in place for an additional 60 seconds. In the NS group, 0.2 µL of normal saline was injected into each side of the CA1 region. Vitamin K (Caspian Tamin, Iran) and Vit K solvent (10 mg/kg s.c. (13) and 1 mL/kg s.c., respectively) were administered daily—once a day—for thirty days. The day after the last injection, and one day later, the rats were exposed to the open-field and passive avoidance apparatus.
3.4. Passive Avoidance Learning
To assess acquisition learning and memory, we used a passive avoidance apparatus consisting of two identically sized light and dark chambers (20 cm × 20 cm × 40 cm) separated by a rectangular opening controlled by a guillotine door (8 cm × 8 cm). Both chambers had a grid floor connected to an electrical stimulator in the dark chamber.
For habituation training, two test trials were conducted. In each trial, rats were placed in the light chamber, and after 10 seconds, the guillotine door was opened. When the rats entered the dark chamber, the door was closed. After 30 seconds, the rats were removed from the dark chamber and returned to their holding cage. The interval between each trial was 30 minutes.
The acquisition trial was conducted 30 minutes later. The entrance latency, or step-through latency (STLa), was defined as the time taken for the rats to enter the dark chamber with all four paws. After entering the dark chamber, the guillotine door was closed, and an electric foot shock (50 Hz square wave, 1 mA) was applied for 1.5 seconds. The rats were then removed from the chamber and placed back in the light chamber for 120 seconds. If the rats re-entered the dark compartment after 120 seconds, the acquisition trial was repeated, and the number of trials (NTa) was recorded (14).
Before testing, the rats were allowed to freely explore the apparatus, moving between the light and dark chambers. This procedure helped reduce the time needed to enter the dark box during testing.
The retention test was conducted the following day. Each rat was placed in the light chamber for 10 seconds, the guillotine door was opened, and the latency to enter the dark compartment (STLr) as well as the time spent in TDC were recorded, with a maximum duration of 600 seconds. No electric shock was administered during this retention test.
3.5. Open-Field Test
The open-field apparatus consisted of a circular metal area with a diameter of 77 cm and a height of 41 cm. The floor was divided into 26 sections marked with black lines, and the top was open and illuminated by room lighting. During the acquisition phase, each rat was placed in the center section and allowed to move freely for 5 minutes. The recall test was conducted in the same manner 24 hours later. In both the acquisition and recall phases, the following behavioral parameters were recorded: Rearing, defined as the number of times the rats stood on their rear paws; grooming, recorded as the number of instances of paw cleaning and face washing; defecation, measured by the number of fecal boli produced; and locomotion activity, noted as the number of sections crossed with all four paws.
3.6. Statistical Analysis
The Kolmogorov-Smirnov test was used to evaluate data normality. For data with a normal distribution, one-way analysis of variance (ANOVA) and the post hoc Tukey test were applied to analyze group differences. For data with a non-normal distribution, the Kruskal-Wallis test was used to compare the groups. To assess intra-group comparisons, the paired t-test was employed for normally distributed data, while the Wilcoxon test, a non-parametric equivalent, was used for data that did not follow a normal distribution. A P-value of <0.05 was considered statistically significant.
4. Results
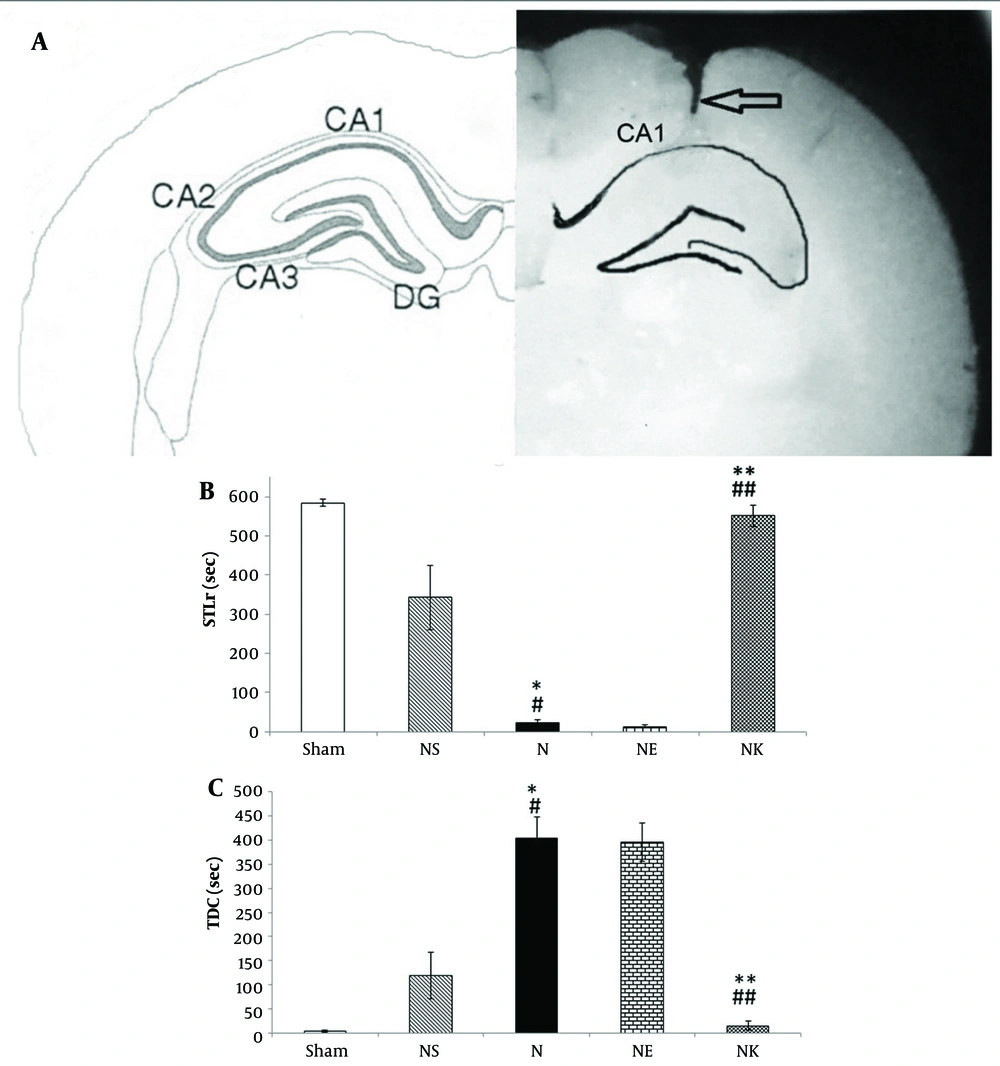
Histological staining of the hippocampal CA1 region with methylene blue confirmed the precise placement of the injection cannula (Figure 1A).
Effects of diferent treatments on the passive avoidance test. A, left, schematic view of hippocampal area of CA1, CA2, CA3 and dentate gyrus (DG). Right, methylene blue-stained area of CA1.The arrow represents the position of the cannula tract; B, the Step-through latency (STLr) in the retention test; C, the time spent in the dark compartment (TDC). Data represents as mean ± S.E.M. NS, normal saline; N, NMDA (N-methyl-D-aspatate); NK, NMDA + Vitamin K (Vit K); NC, NMDA + Vit K solvent (Serum dextrose + Cremophor E1). * P < 0.05 compared to NS group, # P < 0.01 compared to sham group, ** P < 0.05 compared to N group, ## P < 0.01 to compared to NC group (n = 7-8).
4.1. N-methyl-D-aspatate-Induced Memory Impairment in Passive Avoidance Learning
In the initial acquisition trial, there was no difference in STL or the NTa among the Sham, NS, and N groups (data not shown).
The retention test results indicated that the STLr level in the N group was significantly lower than that of the Sham and NS groups (P < 0.001 and P < 0.05, respectively) (Figure 1B). Additionally, the Sham and NS groups exhibited a significantly lower TDC compared to the N group (P < 0.01 and P < 0.05, respectively) (Figure 1C).
4.2. Effect of Vitamin K on N-methyl-D-aspatate-Induced Memory Impairment in Passive Avoidance Learning
The administration of Vit K significantly increased STLr in the NK group compared to the NC group (P < 0.01). Additionally, the NK group showed an increase in STLr when compared to the N group (P < 0.05) (Figure 1B). Furthermore, the NK group demonstrated a lower TDC than both the NC and N groups (P < 0.01) (Figure 1C).
4.3. Effect of Vitamin K on N-methyl-D-aspatate-Induced Memory Impairment in Open-Field Parametersjeanjen
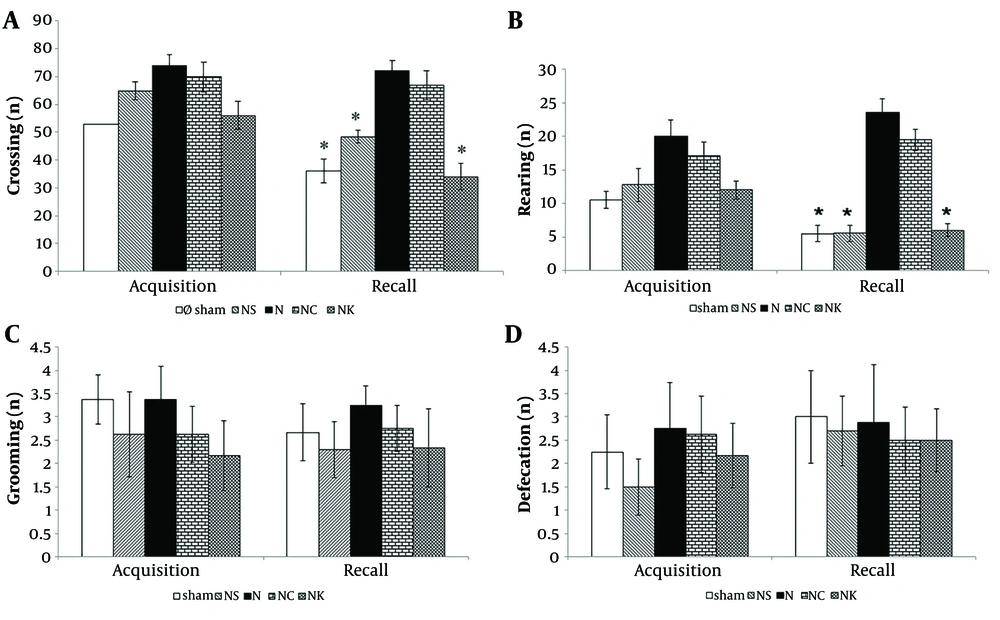
The number of sections crossed and rearing frequency significantly decreased in the Sham and NC groups during recall sessions compared to the acquisition sessions (P < 0.001 for both). However, no significant changes were observed in the sections crossed and rearing frequency in the N group during recall sessions. Administration of Vit K (group NK) led to a significant decrease in both locomotion activity and rearing frequency in the recall sessions (P < 0.001 for both). No statistically significant differences were found in grooming and defecation numbers among the different groups (Figure 2).
Effect of vitamin K (Vit K) on locomotor activity; A, rearing; B, grooming; and C, defecation; D, behavior in the open field. All behaviors were monitored in acquisition and recall sessions, with a 24-hr retention interval. Data represents as mean ± S.E.M. NS, normal saline; N: NMDA (N-methyl-D-aspatate); NK: NMDA + Vit K; NC: NMDA + Vit K solvent (Serum dextrose + Cremophor E1). * P < 0.001 compared with the corresponding group in the acquisition session
5. Discussion
This study evaluated the effect of subcutaneous Vit K injection on rats with NMDA-induced damage in the hippocampal CA1 area using passive avoidance and open-field tests. The results showed that 30 days of Vit K administration significantly increased entrance latency and decreased the time spent in TDC compared to the control group. Additionally, Vit K reduced locomotion activity and the number of rearings in the open-field test.
Learning and memory impairment are key characteristics of neurodegenerative disorders such as Alzheimer’s disease (AD). Glutamate and its action at NMDA receptors play a crucial role in memory (15). Neuronal cell death is induced by NMDA receptor agonists, while antagonists appear to protect neurons (16). Previous studies have confirmed that NMDA injection into the hippocampal CA1 area leads to cell death in this region (1, 16). The toxicity of NMDA is linked to an influx of extracellular Ca2+ through its receptors, directly or indirectly. It is well established that transient changes in intracellular Ca2+ levels can trigger several cellular responses, including apoptosis (17, 18). Furthermore, glutamate activates nuclear factor-κB (NF-κB) through ionotropic glutamate receptors. Nuclear factor-κB activation is associated with neurodegeneration (19) and may contribute to memory impairment (20).
Our results demonstrated that destruction of the hippocampal CA1 area did not affect acquisition but impaired memory retrieval in the passive avoidance test. The lack of memory recall in the acquisition trial during the open-field test suggests that NMDA significantly impaired recognition memory. The observed decrease in rearing numbers and locomotor activity during the recall sessions in the open-field test further indicated impaired recognition memory (21). Although emotional event information may be stored in both the amygdala and hippocampus, these two structures likely encode different aspects of the experience. It has been shown that the amygdala and hippocampus have reciprocal connections, allowing them to modify each other’s responses (22, 23). A study on the roles of hippocampal areas CA1 and CA3 suggested that both regions contribute to acquisition, although only the CA1 region is essential for contextual memory retrieval (1).
In this study, we observed that 30 days of Vit K administration increased the STL and decreased time spent in TDC in the passive avoidance test, indicating an improvement in memory impairment. The ability of Vit K to decrease locomotor activity and rearing behavior in the recall session of the open-field test also reflects enhanced recognition memory. Consistent with our findings, rats on a low-Vit K diet took longer to find the platform in the Morris water maze test compared to those on adequate or Vit K-rich diets (5). In a study on healthy older adults, participants with higher serum Vit K1 levels demonstrated better cognitive function than those with lower levels (24). Furthermore, post-mortem analysis of MK-4 levels in brain tissue indicated that higher MK-4 levels were associated with a reduced likelihood of mild cognitive impairment and fewer neurofibrillary tangles in neuronal cells. This suggests that MK-4 might have a neuroprotective mechanism directly linked to the preservation of cognitive processes and protection against neurofibrillary tangles (25).
Menaquinone-4, the primary form of Vit K in the brains of both young and older rats, plays a role in sphingolipid synthesis (6). Increased MK-4 levels in the brain have shown a positive correlation with sulfatides, cerebrosides, and sphingomyelin but a negative correlation with gangliosides (7). A similar correlation between sulfatide concentrations and MK-4 in the hippocampus has also been observed (8). In contrast, one study indicated that higher lifetime intake of Vit K was associated with lower concentrations of sulfatides and cerebrosides in the brain (7). Cerebrosides, one of the most abundant glycolipids in the myelin sheath and axons, are crucial for nerve function (26, 27). Additionally, sulfatides and cerebrosides regulate myelination and the differentiation of oligodendrocytes (28), with reduced myelin integrity recognized as a key contributor to cognitive deficits (29-31).
Gangliosides, on the other hand, play a role in the protection and repair of nervous tissue (32) and may influence neural cell signaling (33). Meanwhile, Vit K appears to reduce ceramide levels in the hippocampus (7). Ceramide can interfere with neuronal survival pathways (34), and elevated ceramide levels have been associated with AD (35, 36). This suggests that Vit K, through its effects on sphingolipid metabolism, may offer neuroprotective benefits and influence cognitive health.
Vitamin K is known for its neuroprotective effects, particularly through Vit K–dependent proteins (VKDPs) like proteins C and S, and Gas6, which have potent cytoprotective properties. In adult rats, Gas6 is expressed in the brain, including the hippocampus [specifically in areas CA1, CA3, and the dentate gyrus (DG)] (4). Gas6 has shown pro-survival effects on hippocampal neurons by activating MAPK and downstream PI3-K signaling pathways. The Tyro3 receptor, a Gas6 ligand abundant in the hippocampus, appears to play a role in memory consolidation (37, 38) and the maturation of glutamatergic synapses (39). Additionally, Protein S, activated by Vit K, is expressed in the hippocampus and protects neurons from excitotoxic injury (6). The endothelial protein C receptor (EPCR), present on endothelial cells, converts protein C into activated protein C (APC) (4). In hypoxic-ischemic brain injury models, APC treatment has been shown to reduce the number of apoptotic cells in the CA1, CA2, CA3, and DG regions of the hippocampus in both hemispheres, while increasing the number of preserved neurons in CA1 and CA3 (40).
The antioxidant (9) and anti-inflammatory properties of Vit K, as well as its inhibition of the NF-kB signaling pathway (40), may also contribute to preventing neurodegeneration and memory impairment. Recent studies suggest that Vit K supplementation modulates pathways involving NLRP3, caspase-1, and Nrf-2, which are crucial in managing inflammation and oxidative stress. Additionally, Vit K may enhance the expression of tyrosine hydroxylase that contribut in maintaining cognitive functions (41).
A limitation of the present study is the lack of assessment of inflammatory cytokines and oxidative stress biomarkers, which would offer deeper insights into the effect of Vit K on memory impairment.
5.1. Conclusions
Our results demonstrated that a single-dose injection of NMDA into the hippocampal CA1 area increased time spent in TDC and decreased STL in the passive avoidance test, indicating memory impairment. Additionally, NMDA significantly disrupted recognition memory in the open-field test. However, chronic administration of Vit K ameliorated these NMDA-induced memory deficits in rats. Our findings suggest that Vit K may help mitigate excitotoxic neuronal damage and improve memory impairments. Based on these results, we recommend future studies to investigate the mechanisms underlying the therapeutic effects of Vit K on memory improvement, particularly focusing on the role of the CA1 region.