1. Background
Depression is a common but serious disorder in modern society. It can disrupt individuals' normal lives and lead to negative emotions such as sadness, anxiety, frustration, and anger (1, 2). Despite various hypotheses, the underlying pathophysiological mechanisms of depression remain unclear. Since the cytokine hypothesis was proposed in 1991, subsequent evidence has suggested an immunological basis for depression (3-5).
The activation of immune-inflammatory pathways, particularly the release of proinflammatory cytokines (e.g., interleukin-6 (IL-6), interleukin-1β (IL-1β), and TNFα), has been shown to induce neurochemical changes and generate neuroendocrine stimuli that contribute to depression (6-8). Evidence has demonstrated that depressive symptoms can be alleviated through specific anti-inflammatory therapies (9). Chronic systemic elevation of TNF-α, IL-6, and C-reactive protein (CRP), which contribute to low-grade inflammation, is frequently associated with depression (10).
Nitric oxide (NO) appears to play a significant role in the pathogenesis of depression (11). Dysregulation in NO signaling pathways may contribute to the development of this condition (12). Moreover, the disruption of NO signal regulation, along with the downstream production of reactive nitrogen species (RNS), reactive oxygen species (ROS), and inflammatory cytokines, has been implicated in the pathogenesis of type 2 diabetes, atherosclerosis, acute renal injury, and heart disease (13). Elevated concentrations of NO-derived metabolites and the upregulation of enzymes responsible for NO production, particularly the inducible isoform (iNOS), have significant roles in the treatment of major depressive disorder (MDD) (13).
Lipopolysaccharide (LPS), a pro-inflammatory endotoxin, is known to activate microglia and the immune system (14). Furthermore, LPS induces behavioral disturbances that mimic the clinical symptoms of human depression (14). Consequently, LPS can be used as a model for central nervous system (CNS) inflammation, as it promotes depressive-like behaviors. Nuclear factor-κB (NF-κB), a key transcription factor, plays a pivotal role in activating numerous genes in response to inflammation. Upon stimulation by LPS, NF-κB translocates to the nucleus, where it regulates the expression of inflammatory cytokines such as IL-6, TNF-α, and IL-1β (15, 16).
Antidepressant drugs include norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs), and tricyclic antidepressants (TCAs). However, due to the high cost of these medications and their numerous side effects, herbs and natural compounds have gained attention as alternatives (17). Herbal remedies derived from plants and natural products can be effective treatments with fewer side effects than chemical drugs. Plant-derived products have been shown to possess anti-cancer, anti-inflammatory, and antioxidant properties, offering numerous benefits for human health (18-21). Herbal extracts, being rich in antioxidants, serve as effective preventive treatments against various diseases, including depression, by protecting tissues from damage caused by free radicals (14, 22, 23).
Citrus medica L., commonly known as citron, belongs to the citrus family. The fruits of this large plant feature a thin yellow peel and contain several active compounds, primarily flavonoids, along with coumarins and terpenoids. Citrus medica exhibits a range of biological activities, including anti-inflammatory, antioxidant, antibacterial, analgesic, anti-tumor, anti-cancer, anti-cholinesterase, anti-Alzheimer, and hypoglycemic effects (24, 25). Some flavonoids structurally resemble inhibitors of cellular signaling cascades and effectively suppress the expression of iNOS and NO production in activated glial cells (26). Flavonoids also inhibit the secretion of pro-inflammatory cytokines, cytokine-induced NO production, NADPH (nicotinamide adenine dinucleotide phosphate) activation, and reactive oxygen species (ROS) production. Their mechanism of action partly involves inhibiting the NF-κB signaling pathway and mitigating neuroinflammation (27).
2. Objectives
Despite substantial evidence suggesting the potential protective role of C. medica against pathological conditions of the central nervous system, the precise mechanisms by which this herbal medicine acts on depression remain unclear. In the present study, we aimed to explore the therapeutic effects of C. medica extract and the potential involvement of the NO pathway in a mice model of depression induced by LPS.
3. Methods
3.1. Chemicals
Escherichia coli LPS, L-Nitro arginine methyl ester (L-NAME), Aminoguanidine (AG), and L-arginine (L-Arg) were purchased from Sigma-Aldrich (Buchs, Switzerland).
3.2. Animals
Male Swiss albino mice (8 weeks old, weighing 23 ± 2 g) were obtained from Elm Bavarian Aftab Company, Kermanshah, Iran. The mice were housed under controlled conditions of 25 ± 1°C, with a 12-hour light/12-hour dark cycle, and had free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee at Kermanshah University of Medical Sciences (IR.KUMS.REC.1400.235).
3.3. Extraction Preparation
To prepare the extract, 1 kg of C. medica fruit was dried at 25 - 30°C and ground into a fine powder. A total of 100 g of this powder was mixed with 300 mL of distilled water and 700 mL of 97% ethanol (v/v) and stirred continuously on a magnetic stirrer at room temperature for 48 hours. Afterward, the solvent-plant mixture was allowed to settle at room temperature for 1 hour, followed by filtration through Whatman filter paper No. 1 (28). The filtered extract was transferred to a 1000 cc round-bottom flask, and the solvent was evaporated using a rotary evaporator. Finally, the concentrated extract was transferred to a watch glass for further concentration and storage (29).
3.4. Experimental Design
Mice were divided into 9 experimental groups (n = 7 mice per group): (1) Control group: Received a standard diet; (2) LPS group: Injections of LPS (1 mg/kg/day) (30) to induce depression; (3) fluoxetine (FLX) group: Injections of fluoxetine (20 mg/kg) (31) as the positive control (30 minutes before LPS injection); (4 - 6) CM group: Injections of CM extract at 3 doses (250, 500, and 750 mg/kg, respectively) 30 minutes before LPS injection; (7) combined treatment of CM 500 mg/kg + L-Arginine (750 mg/kg) (32) group (30 minutes before LPS injection); (8) combined treatment of 250 mg/kg CM + L-NAME (10 mg/kg) (33) group (30 minutes before LPS injection); (9) Combined treatment of CM 250 mg/kg + AG (50 mg/kg) (34) group (30 minutes before LPS injection). Behavioral tests were conducted 24 hours after the administration of LPS. The LPS dose (1 mg/kg) was chosen based on previous studies evaluating depressive-like behavioral alterations and neurochemical changes in mice (35). The doses of CM (250, 500, and 750 mg/kg) were selected based on a preclinical study evaluating its antidepressant-like activity (36). Fluoxetine was used as a standard antidepressant (37). The doses of L-NAME and AG were selected because they have no antidepressant effect when used alone (38).
3.5. Forced Swimming Test
The forced swimming test (FST) is one of the most commonly used animal tests for depression (39). Mice are placed in an inescapable transparent tank filled with water, and their escape-related mobility behavior is measured. The tank (25 cm long, 12 cm wide, and 15 cm high) is filled with water at 25°C, and the animal is gently placed into the water from a height of 20 cm. Cessation of limb movements by the mouse is considered immobilization. This test was repeated three times with a 1-minute break between trials. The cut-off time was 6 minutes.
3.6. Tail Suspension Test
The tail suspension test (TST) is another classic model used to assess the efficacy of antidepressants (40). In this method, metal bases with a height of 70 cm are used. The mice are hung from a height of 50 cm, with their tails fixed using glue to a designated point. For 6 minutes, the immobility time of the mice is measured with a chronometer, and this is considered an indicator of depression. This test was repeated three times with a 1-minute break between trials.
3.7. Open Field Test
The open field test (OFT) was performed to evaluate the locomotor activity of mice (41). In this test, an open box (40 × 60 × 50 cm) was used, and its floor was divided into several equal sections by lines. The animal was randomly placed in the center of the box and allowed to move freely for 10 minutes. The number and duration of movements were measured as indicators of motor activity. The amount of time the mice spent in the central square was considered a non-depressive factor. As with the previous two tests, this procedure was repeated three times with a 1-minute interval between trials.
3.8. TNF-α Determination
After performing the behavioral tests, the mice were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg), and the entire brain tissue was dissected and stored at -25°C until TNFα levels were measured. The brain tissue was homogenized in normal saline, centrifuged at 12,000 rpm for 20 minutes at 4°C, and the supernatant was immediately used to measure TNFα. The assessment of the inflammatory factor TNFα was performed using a commercial TNFα assay kit (Catalog No: KPG-MTNFP). Fifty μL of standards or samples were added to the wells, and the plate was placed on a shaker (RPM200) for 1 hour. Then, the plate was washed three times, followed by the addition of 50 μL of conjugated antibody to all wells, and incubation was repeated on the shaker for 1 hour. After washing, 50 μL of diluted HRP-avidin was added to each well, and incubation was carried out for 30 minutes at room temperature on a shaker. After five washes, 50 μL of substrate was added, and the plate was incubated for an additional 15 minutes. Finally, 25 μL of stop solution was added, and the absorbance was measured using a 96-well microplate reader (CLARIOStar, Germany) at a wavelength of 450 nm.
3.9. Data Analysis
Data analysis was performed using SPSS software version 25. All data are presented as Mean ± SEM. One-way analysis of variance (ANOVA) was conducted, followed by the Tukey post-hoc test for group comparisons. A P-value < 0.05 was considered statistically significant.
4. Results
4.1. Behavioral Tests
4.1.1. Evaluation of the Effect of Citrus medica on Immobility Time in the Open Field Test
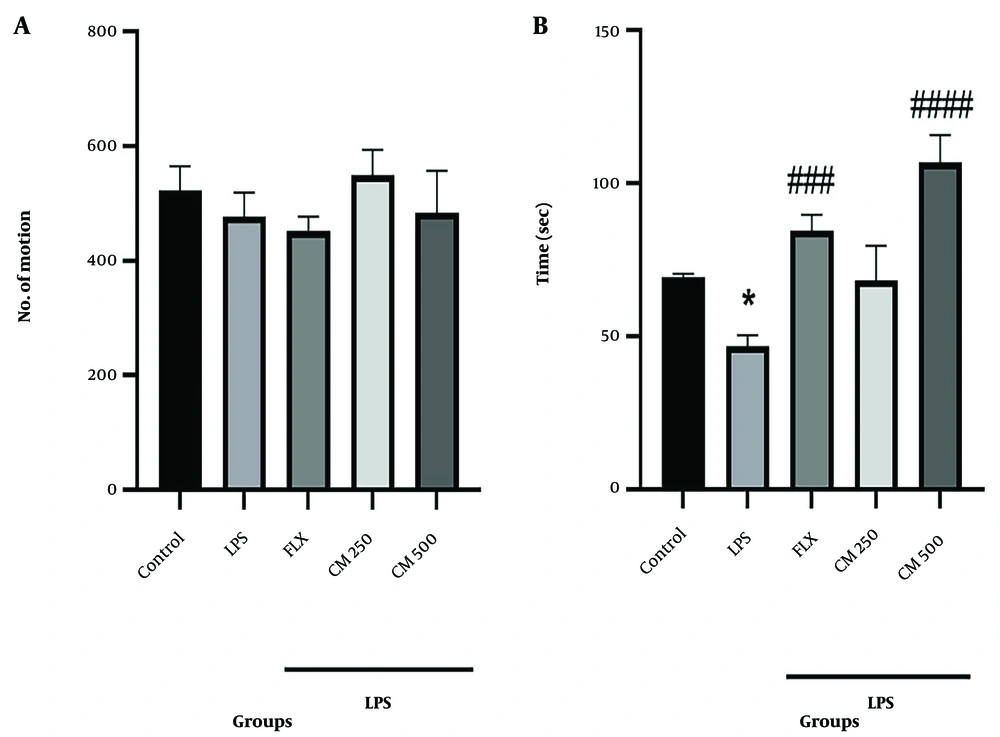
Figure 1A shows the number of movements and the amount of physical activity of the mice in the OFT. The results indicated no significant differences in the number of movements between any of the groups, suggesting that neither LPS, CM, nor FLX administration affected the locomotor activity. Figure 1B shows that the time spent by the mice inside the square was reduced in the LPS group compared to the control group (P < 0.05). However, this time was progressively increased in the groups that received the CM extract compared to the LPS group, although the differences were only statistically significant for the 500 mg/kg group (#### P < 0.0001).
4.1.2. Evaluation of the Effect of Citrus medica on Immobility Time in the Forced Swimming Test and Tail Suspension Test
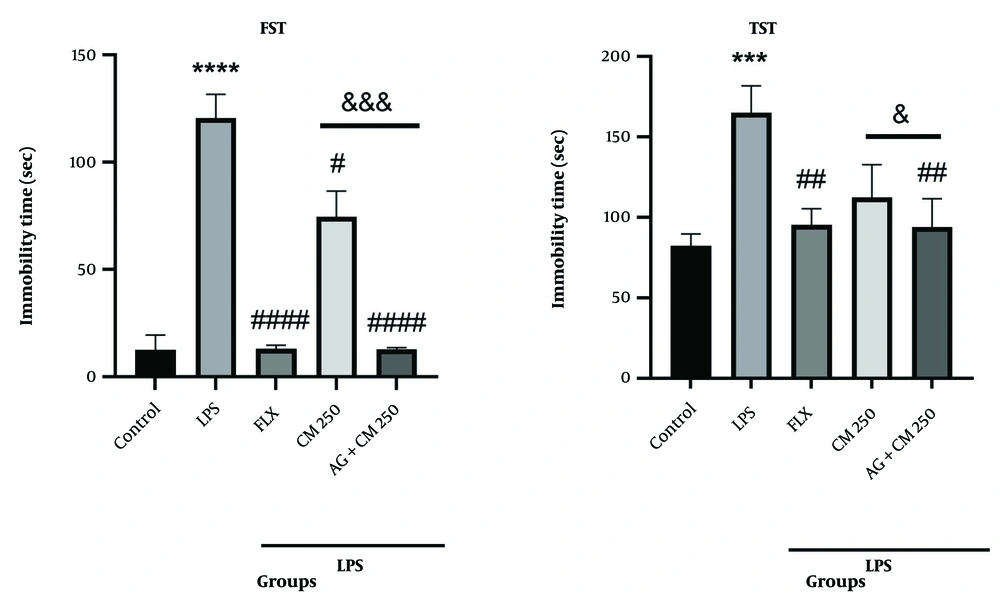
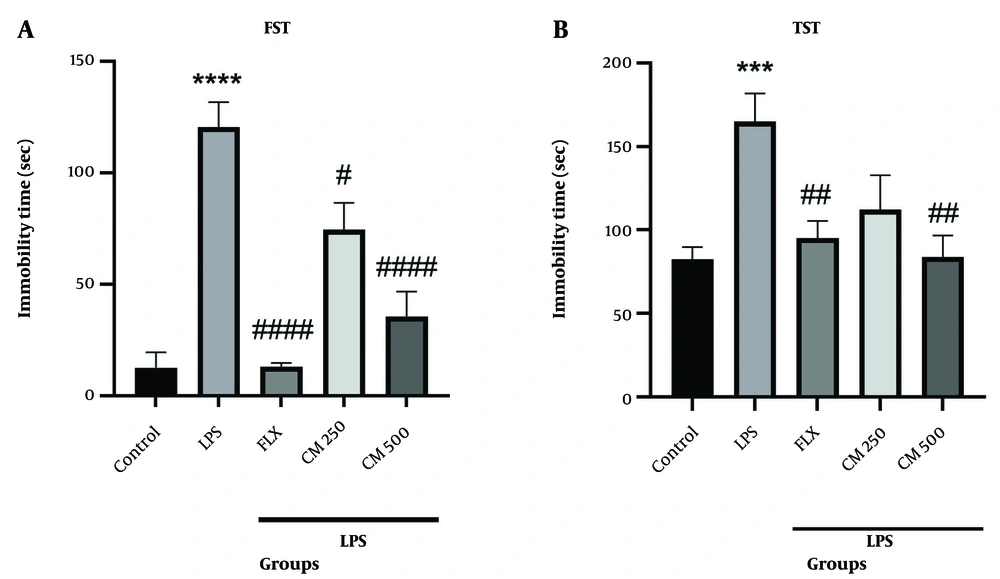
Figure 2A shows that the immobility time in the LPS group was significantly increased compared to the control group (P < 0.0001). The groups that received the CM extract (250 and 500 mg/kg) and FLX showed a significant reduction in immobility time compared to the LPS group (# P < 0.05, #### P < 0.0001, and #### P < 0.0001, respectively). Changes in immobility time were also observed in the TST test. The immobility time in the LPS group increased significantly compared to the control group (P < 0.001). Treatment with CM extract (500 mg/kg) and FLX reversed the results compared to the LPS group (## P < 0.01). No significant difference was observed between the 250 mg/kg extract group and the LPS group (Figure 2B).
Behavioral tests forced swimming test (FST) and tail suspension test (TST) (n = 7). A, the immobility time in the FST. **** P < 0.0001 vs. the control group; # P < 0.05, and #### P < 0.0001 vs. the lipopolysaccharide (LPS) group; B, the duration of immobility in the TST. *** P < 0.001 vs. the control group; ## P < 0.01 vs. the LPS group.
4.1.3. Effect of Citrus medica (500 mg/kg) Extract in Combination with L-Arginine (750 mg/kg) on Lipopolysaccharide-Induced Depression in the Open Field Test
No significant difference in the number of movements was observed between any of the groups, suggesting the absence of locomotor activity disorder (Figure 3A). In the LPS and L-Arg groups, the time spent in the center of the field was significantly reduced compared to the control group (P < 0.05). Administration of L-Arg 15 minutes before the injection of CM extract (500 mg/kg) significantly ameliorated the effects of CM extract when administered alone. Specifically, in the L-Arg group with CM extract (500 mg/kg), the time spent in the center was significantly decreased compared to the CM-only group (&&& P < 0.001) (Figure 3B).
Behavioral test [open field test (OFT)] (n = 7). A, shows the activity of the mice. There is no significant difference in the number of movements or activity between thecontrol, lipopolysaccharide (LPS), L-Arg, and CM groups; B, shows the time spent in the center. Lipopolysaccharide and both L-Arg groups compared to the control group (* P < 0.05). L-Arg group with CM extract (500 mg/kg) compared to the CM group alone (&&& P < 0.001). Fluoxetine (FLX) and CM 500 groups compared to the LPS group (### P < 0.001, #### P < 0.0001, respectively).
4.1.4. Effect of Citrus medica (500 mg/kg) Extract in Combination with L-Arginine on Lipopolysaccharide-Induced Depression in the Forced Swimming Test and Tail Suspension Test
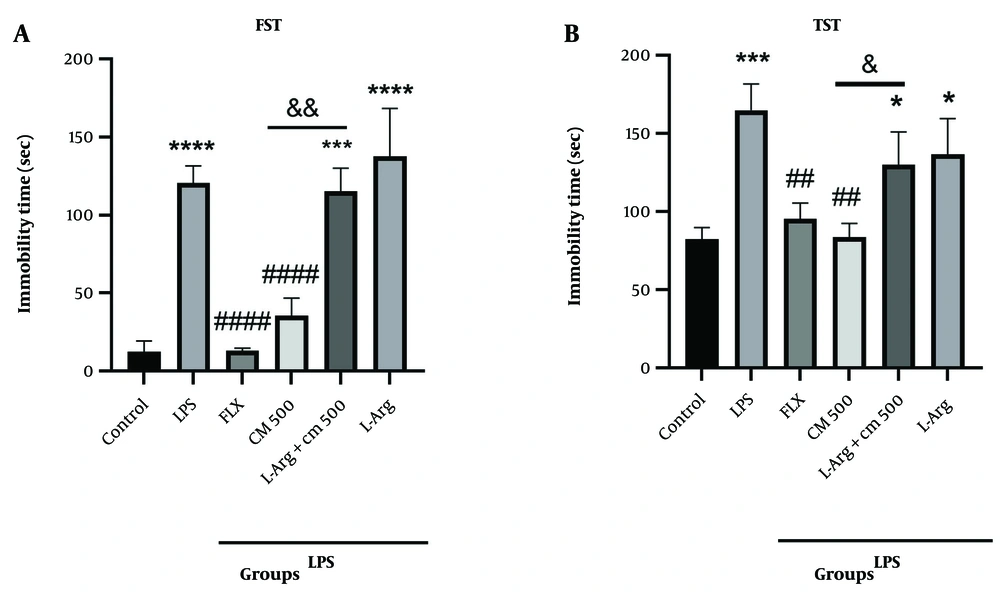
As shown in Figure 4A, the immobility time in the LPS group was significantly increased compared to the control group (P < 0.0001). L-Arg induced depressive-like behavior, elevating the immobility time in the FST compared to the control group (P < 0.0001). Administration of L-Arg 15 minutes before the injection of CM extract (500 mg/kg) significantly reversed the immobility time compared to the CM extract alone (&& P < 0.01). In the TST (Figure 4B), the immobility time in the LPS group was increased compared to the control group (P < 0.001). Administration of L-Arg 15 minutes before the injection of CM extract (500 mg/kg) increased the immobility time compared to the CM extract alone (& P < 0.05).
Behavioral tests forced swimming test (FST) and tail suspension test (TST) (n = 7). A, shows the immobility time in the FST. *** P < 0.001 and **** P < 0.0001 vs. the control group; #### P < 0.0001 vs. the lipopolysaccharide (LPS) group. L-Arg group with CM extract (500 mg/kg) compared to the CM group alone (&& P < 0.01); B, shows the immobility time inthe TST. * P < 0.05 and *** P < 0.001 vs. the control group; ## P < 0.01 vs. the LPS group. L-Arg group with CM extract (500 mg/kg) compared to the CM group alone (& P < 0.05).
4.1.5. Effect of Citrus medica (250 mg/kg) Extract in Combination with L-NAME (10 mg/kg) on Lipopolysaccharide-Induced Depression in the Open Field Test
The results obtained from the OFT (Figure 5A) showed no significant difference in the number of movements between groups, suggesting the absence of locomotor activity disorders. However, the duration of time spent by the mice inside the square was reduced in the LPS group compared to the control group (P < 0.05). Figure 5B indicates that the administration of L-NAME 15 minutes before the injection of CM extract (250 mg/kg) significantly increased the anti-depressive effect of CM, as the mice spent more time in the center of the square compared to the CM-only group (&&& P < 0.001).
Behavioral test [open field test (OFT)]. A, represents the number of movements of the mice. There is no significant difference in the number of movements or activitybetween the control, lipopolysaccharide (LPS), L-NAME, and Citrus medica groups; B, shows the time spent in the center. Lipopolysaccharide vs. the control group (* P < 0.05). Fluoxetine (FLX) group vs. LPS alone group, the L-NAME group with CM extract (250 mg/kg) vs. the CM alone and LPS groups (### P < 0.001, #### P < 0.0001, &&& P < 0.001, respectively).
4.1.6. Effect of Citrus medica (250 mg/kg) Extract in Combination with L-NAME (10 mg/kg) on Lipopolysaccharide-Induced Depression in the Forced Swimming Test and Tail Suspension Test
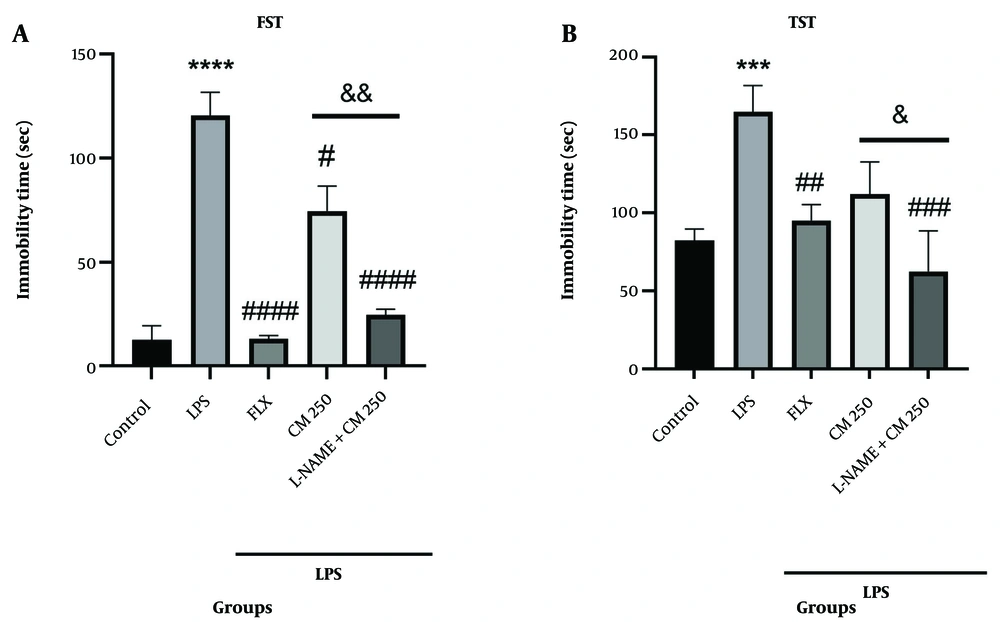
The immobility time in the LPS group was significantly increased compared to the control group (P < 0.0001) (Figure 6A). Administration of L-NAME 15 minutes before the injection of CM extract (250 mg/kg) significantly decreased the immobility time in the CM extract group compared to the CM-only group in the FST (&& P < 0.01). As shown in Figure 6B, the immobility time in the LPS group was increased compared to the control group (P < 0.001). Administration of L-NAME along with CM significantly reduced the immobility time compared to the CM-only group (& P < 0.05).
Behavioral tests. A, shows the immobility time in the forced swimming test (FST). **** P < 0.0001 vs. the control group; # P < 0.05, #### P < 0.0001 vs. the lipopolysaccharide (LPS) group. The L-NAMEgroup with CM extract (250 mg/kg) vs. the CM group alone (&& P < 0.01); B, shows the immobility time in the tail suspension test (TST). *** P < 0.001 vs. the control group; ## P < 0.01 and ### P < 0.001 vs. the LPS group. The L-NAME group with CM extract (250 mg/kg) vs. the CM group alone (& P < 0.05).
4.1.7. Effect of Citrus medica (250 mg/kg) Extract in Combination with Aminoguanidine (AG) (50 mg/kg) on Lipopolysaccharide-Induced Depression in the Open Field Test
The results from the OFT (Figure 7A) showed no significant difference in the levels of physical activity between the groups, indicating no impairment of locomotor activity. Figure 7B shows that the duration of time spent by the mice inside the square was reduced in the LPS group compared to the control group (P < 0.05). Administration of AG 15 minutes before the injection of CM extract (250 mg/kg) significantly enhanced the anti-depressive effect of CM. Specifically, in the CM + AG group, mice spent more time in the center of the square compared to the CM-only group (& P < 0.05).
Behavioral test [open field test (OFT)] (n = 7). A, Shows the number of movements of the mice. There is no significant difference in the number of movements or activitybetween the control, lipopolysaccharide (LPS), AG + CM, and CM groups; B, shows the time spent in the center. LPS vs. the control group (* P < 0.05). The AG group with CM extract (250 mg/kg) vs. the CM alone and LPS groups (& P < 0.05 and ### P < 0.001, respectively). Fluoxetine (FLX) group vs. LPS group (### P < 0.001).
4.1.8. Effect of Citrus medica (250 mg/kg) Extract in Combination with Aminoguanidine (AG) (50 mg/kg) on Lipopolysaccharide-Induced Depression in the Forced Swimming Test and Tail Suspension Test
Figure 8A shows that the immobility time in the LPS group was significantly increased compared to the control group (P < 0.0001). AG administration significantly decreased the immobility time in the FST compared to the control group (P < 0.0001). Administration of AG 15 minutes before the injection of CM extract (250 mg/kg) significantly enhanced the anti-depressive effect of CM extract compared to the CM-only group (&&& P < 0.001). In the TST (Figure 8B), the immobility time in the LPS group was increased compared to the control group (P < 0.001). Administration of AG 15 minutes before the injection of CM extract (250 mg/kg) reduced the immobility time in the CM + AG group compared to the CM-only group (& P < 0.05).
Behavioral tests forced swimming test (FST) and tail suspension test (TST) (n = 7). A, shows the immobility time in the FST. **** P < 0.0001 vs. the control group; # P < 0.05, #### P < 0.0001 vs. the lipopolysaccharide (LPS) group. The AG group with CM extract (250 mg/kg) vs. the CM group alone (&&& P < 0.001); B, shows the immobility time in the TST. *** P < 0.001 vs. the control group; ## P < 0.01 vs. the LPS group. The AG group with CM extract (250 mg/kg) vs. the CM group alone (& P < 0.05). LPS group vs. the control group in FST and TST tests (**** P < 0.0001, *** P < 0.001 respectively).
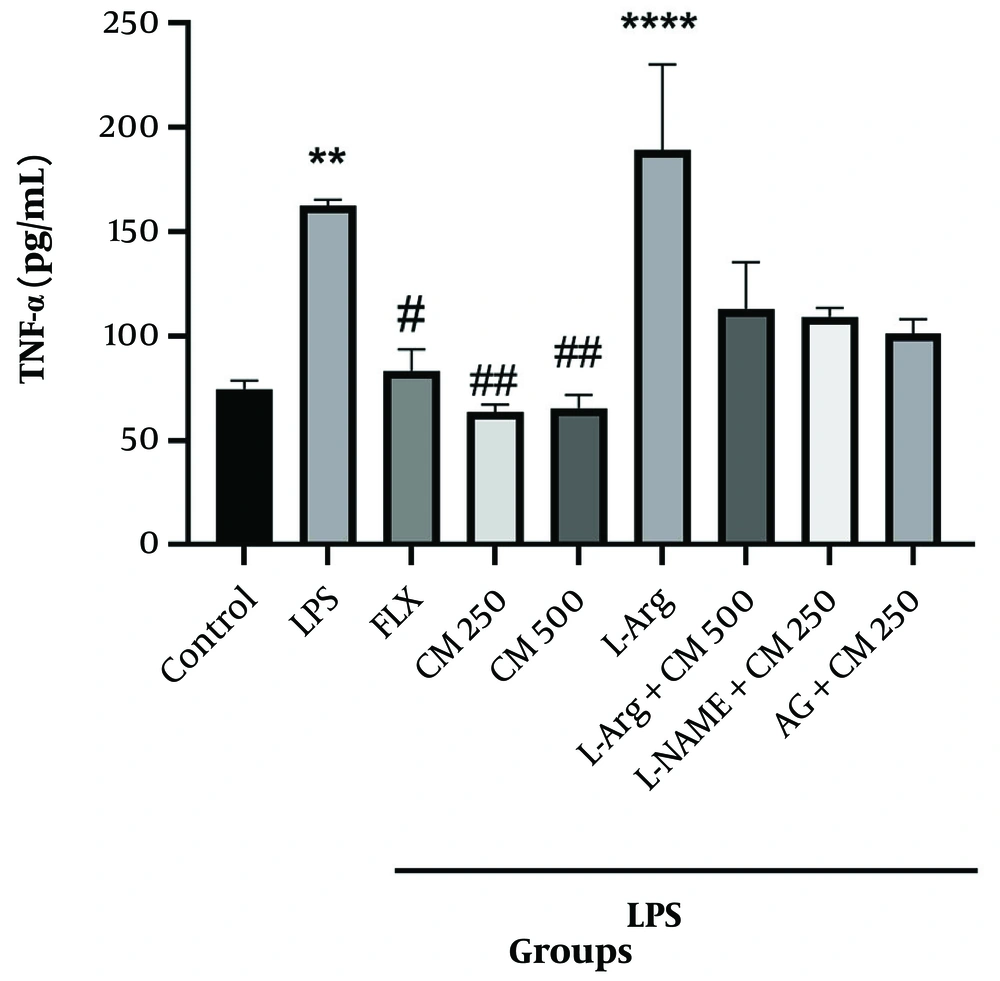
4.2. Effect of Different Doses of Citrus medica Extract on Brain TNF-α Levels in Depressive Mice
TNF-α levels in the LPS and L-Arg groups significantly increased (P < 0.01 and P < 0.0001, respectively) compared to the control group. In contrast, TNF-α levels in the groups that received L-Arg along with CM extract (500 mg/kg) did not show significant differences compared to the CM extract (500 mg/kg) group alone (P > 0.05). Similarly, in the L-NAME and AG groups with CM extract (250 mg/kg), compared to the CM extract (250 mg/kg) group alone, no significant difference was observed (P > 0.05) (Figure 9).
5. Discussion
Depression is a common mental disorder that affects the health and quality of life of patients. It is associated with psychological, social, and physical problems, as well as an increased risk of suicide (42). Decreased levels of monoamines (serotonin, norepinephrine, and dopamine) in the brain are among the primary causes of depression (17). Inflammatory processes are also involved in the pathophysiology of behaviors related to depression and anxiety in animal models (43). In addition to inflammation and decreased levels of monoamines, NO plays an important role in the pathophysiology of various disorders in the organism (44, 45). Numerous studies have reported the involvement of NO in the pathogenesis of depression. Nitric oxide modulates norepinephrine, serotonin, dopamine, and glutamate, which are major neurotransmitters involved in the pathology of depression (44). Sekio and Seki have shown that increases in IL-6 and TNF-α levels in the blood are associated with depression (46). Recent studies have also shown that the administration of LPS can cause depressive symptoms in rodents by increasing pro-inflammatory cytokines through the N-methyl-D-aspartate (NMDA) receptor (47, 48). Available medications for depression, such as monoamine oxidase inhibitors, tricyclic antidepressants, and selective serotonin reuptake inhibitors, are associated with a wide range of side effects affecting sleep, the gastrointestinal tract, and the nervous system. Therefore, there is a need for research into effective drugs with fewer side effects (49, 50). Researchers have found that natural compounds, such as C. medica, with anti-inflammatory and antioxidant activity, could be promising agents to prevent neuronal death and central nervous system dysfunction (51-53).
Citrus medica is known for its strong antioxidant properties and its effectiveness in reducing inflammation and pain in mice (36). The antioxidant activity may be attributed to the presence of vitamin C and phenolic compounds (54). The results of Lim et al.'s study have shown that the extract of Citrus unshiu peel plays an effective role in improving depressive-like behaviors and neurotoxicity caused by dexamethasone in mice (55). In 2017, Braidy et al. investigated the neuroprotective effects of Citrus and indicated that its flavonoids act as important neuroprotective agents against oxidative stress. These flavonoids regulate several signaling pathways, including mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinases (ERK1/2), protein kinase A (PKA), protein kinase B (PKB), and protein kinase C (PKC) (56). Michini et al. (as cited by Jafarpour et al.) showed that immature citron fruit has significant antioxidant properties and inhibits alpha-glucosidase activity. It has also been shown that citron leaf extract has antioxidant activity and an inhibitory effect on NO production (57). Potential compounds, such as polyphenols and bioflavonoids, have major effects on inflammatory pathways and NO activity (57). Flavonoids such as quercetin and the anthocyanin malvidin are found in plants including tea, fruit juices, wine, and grapes. Studies show that flavonoids can trigger a neurological response (58). By inhibiting oxidative stress and neuroinflammation, regulating neurotransmitter levels, increasing synaptic plasticity, and stimulating brain-derived neurotrophic factor signaling, flavonoids have been introduced as suitable candidates for the treatment of anxiety and depression (59).
The results of an in vitro study showed that CM extract could play an effective role in eliminating various free radicals (54). A study by Kim et al. demonstrated that the essential oil of CM was capable of inhibiting the expression of prostaglandin E2 (PGE2), cyclooxygenase 2 (COX-2), NO, and iNOS. Additionally, it inhibited LPS-induced inflammation by blocking TNF-α, NF-κB, c-Jun N-terminal kinase (JNK), and extracellular-signal-regulated kinase (ERK) pathways in macrophages (60). The LPS induces the production and release of cytokines, subsequently causing symptoms that resemble depression, which makes it useful in models of depression (61). The results of this study showed that CM extract at a dose of 500 mg/kg could be effective in improving depressive behaviors induced by LPS in mice and reducing the duration of immobility. Nitric oxide is synthesized from L-Arginine by a family of isoenzymes called NOS, with iNOS being the most relevant due to its inducible character (62). The results of the present study showed that the injection of 750 mg/kg L-Arginine exacerbated depressive-like behaviors in mice as observed in the FST and TST. The administration of 500 mg/kg CM extract reduced the duration of immobility in mice in the FST compared to the L-Arg group alone, demonstrating the capability of the extract to improve depressive-like behaviors in mice.
To accurately investigate the possible role of the NO pathway, FST and TST tests were conducted, combining CM extract with some NOS inhibitors (L-NAME, aminoguanidine). The inhibitors did not show a significant antidepressant effect, but when combined with CM extract (in a low, ineffective dose), they enhanced the antidepressant effects of the extract (63). L-NAME is used as a non-specific inhibitor of NOS in various biological systems (64). The outcomes of an in vivo study revealed that auraptene, at a dose of 30 mg/kg, improved depressive-like behaviors, decreased serum NO levels, increased serum MDA, and enhanced serum antioxidant capacity. Additionally, the co-administration of L-NAME with auraptene (ineffective dose) increased the effects of auraptene (65). In the present study, we showed that the combination of L-NAME with CM extract reduced the time of immobility compared to the extract group alone and improved depressive-like behaviors in mice. Aminoguanidine is known as a specific inhibitor of iNOS (66) and also leads to the inhibition of inflammation (67). This enzyme plays an important role in the excessive production of oxidative and nitrosative stress and the activation of NF-κB, which triggers inflammatory and oxidative processes, leading to subsequent neurotoxicity (68). The present study showed that the administration of AG with CM extract (250 mg/kg) reduced immobility compared to the CM extract alone. These results provide evidence of the potential therapeutic effect of AG and its ability to improve LPS-induced depressive behaviors (68). The inhibition of iNOS could intensify the effects of the extract, assuming that the extract exerts its effect by inhibiting NO production. These findings also suggest the role of iNOS in the antidepressant effect of the extract.
TNF-α, as an inflammatory mediator, is widely used to evaluate the inflammatory course of a disease or the inflammatory or anti-inflammatory effects of a drug (69, 70). The results showed that in the LPS and L-Arginine groups, TNF-α levels were significantly increased, reaching values greater than 150 pg/mL, indicating the severity of inflammation. However, in the groups receiving 250 mg/kg and 500 mg/kg of the extract, TNF-α levels were significantly decreased compared to the control and fluoxetine groups, evidencing the anti-inflammatory effects of the extract.
5.1. Conclusions
Depression and anxiety, as chronic, common, and debilitating mental disorders, are among the most critical diseases in the global health system. The chemical drugs used to treat these diseases have side effects, so the use of medicinal plants is an interesting approach to their treatment. Medicinal plants are considered more desirable than chemical drugs due to their greater popularity, availability, and fewer side effects. The results of the present study showed that CM extract exerted antidepressant effects in a mouse model, and its effects were mediated, at least in part, by modulating the NO signaling pathway and reducing the production of the pro-inflammatory cytokine TNF-α. The extract reduced the production of NO, likely by inhibiting NOS, as evidenced by the use of specific enzyme inhibitors. Based on these outcomes, it can be argued that CM could be a promising candidate for the treatment of brain disorders such as depression.
![Behavioral test [open field test (OFT)] (n = 7). A, shows the activity of the mice. There is no significant difference in the number of movements or activity between thecontrol, lipopolysaccharide (LPS), L-Arg, and CM groups; B, shows the time spent in the center. Lipopolysaccharide and both L-Arg groups compared to the control group (* P < 0.05). L-Arg group with CM extract (500 mg/kg) compared to the CM group alone (&&& P < 0.001). Fluoxetine (FLX) and CM 500 groups compared to the LPS group (### P < 0.001, #### P < 0.0001, respectively). Behavioral test [open field test (OFT)] (n = 7). A, shows the activity of the mice. There is no significant difference in the number of movements or activity between thecontrol, lipopolysaccharide (LPS), L-Arg, and CM groups; B, shows the time spent in the center. Lipopolysaccharide and both L-Arg groups compared to the control group (* P < 0.05). L-Arg group with CM extract (500 mg/kg) compared to the CM group alone (&&& P < 0.001). Fluoxetine (FLX) and CM 500 groups compared to the LPS group (### P < 0.001, #### P < 0.0001, respectively).](https://services.brieflands.com/cdn/serve/3170b/b3a355126787a0a0faea9fb2e9e717499dfdc6c5/jrps-148619-i003-F3-preview.webp)
![Behavioral test [open field test (OFT)]. A, represents the number of movements of the mice. There is no significant difference in the number of movements or activitybetween the control, lipopolysaccharide (LPS), L-NAME, and <i>Citrus medica</i> groups; B, shows the time spent in the center. Lipopolysaccharide vs. the control group (* P < 0.05). Fluoxetine (FLX) group vs. LPS alone group, the L-NAME group with CM extract (250 mg/kg) vs. the CM alone and LPS groups (### P < 0.001, #### P < 0.0001, &&& P < 0.001, respectively). Behavioral test [open field test (OFT)]. A, represents the number of movements of the mice. There is no significant difference in the number of movements or activitybetween the control, lipopolysaccharide (LPS), L-NAME, and <i>Citrus medica</i> groups; B, shows the time spent in the center. Lipopolysaccharide vs. the control group (* P < 0.05). Fluoxetine (FLX) group vs. LPS alone group, the L-NAME group with CM extract (250 mg/kg) vs. the CM alone and LPS groups (### P < 0.001, #### P < 0.0001, &&& P < 0.001, respectively).](https://services.brieflands.com/cdn/serve/3170b/1d905f3b629f802f9e35f568407fa50799e09524/jrps-148619-i005-F5-preview.webp)
![Behavioral test [open field test (OFT)] (n = 7). A, Shows the number of movements of the mice. There is no significant difference in the number of movements or activitybetween the control, lipopolysaccharide (LPS), AG + CM, and CM groups; B, shows the time spent in the center. LPS vs. the control group (* P < 0.05). The AG group with CM extract (250 mg/kg) vs. the CM alone and LPS groups (& P < 0.05 and ### P < 0.001, respectively). Fluoxetine (FLX) group vs. LPS group (### P < 0.001). Behavioral test [open field test (OFT)] (n = 7). A, Shows the number of movements of the mice. There is no significant difference in the number of movements or activitybetween the control, lipopolysaccharide (LPS), AG + CM, and CM groups; B, shows the time spent in the center. LPS vs. the control group (* P < 0.05). The AG group with CM extract (250 mg/kg) vs. the CM alone and LPS groups (& P < 0.05 and ### P < 0.001, respectively). Fluoxetine (FLX) group vs. LPS group (### P < 0.001).](https://services.brieflands.com/cdn/serve/3170b/1b343e58a34f3e4b4974ba2b3a053605200b2701/jrps-148619-i007-F7-preview.webp)