1. Background
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn's disease, is an idiopathic recurrent bowel disorder (1). Dysregulation in the secretion and synthesis of pro-inflammatory cytokines, leukocyte infiltration, and unbalanced autophagy leads to inflammation, ulcerative lesions, diarrhea or constipation, abdominal pain, and fever in patients with UC (1). Conventional treatment for UC involves aminosalicylates and corticosteroids, as well as alternative therapies, immunosuppressive compounds, and biological response modifiers (2). Unfortunately, these drugs do not provide a cure, and patients may experience recurrent severe episodes and significant adverse effects (2). Consequently, further research is needed to identify novel therapeutic approaches for treating UC.
The increasing interest in herbal medicines has led to numerous studies evaluating the beneficial effects of medicinal plants in UC (3). Allium cepa L. (common onion), a biennial plant of the Liliaceae family, is cultivated worldwide and used as both a vegetable and a flavoring agent (4). In addition to its nutritional value, organosulfur, anthocyanin, flavonoids, quercetin, kaempferol, and polyphenols are the main compounds in onion outer skin, contributing to its antioxidant and anti-inflammatory properties (4, 5). Red onion peels (A. cepa), often discarded in large quantities by the food industry, contain high levels of quercetin, the main flavonoid (6). Evaluating onion peel as a rich natural source of functional compounds could reveal its beneficial health effects, particularly in chronic diseases.
2. Objectives
While some previous studies have demonstrated the advantageous anti-inflammatory effects of onion bulb extract in various experimental UC models (7-9), the primary objective of the present study is to investigate the potential benefits of red onion peel extract in the acetic acid UC model. Additionally, we conducted further analysis to identify the active components of A. cepa L. peel methanolic extract (ACPME) responsible for attenuating inflammation.
3. Methods
3.1. Chemicals and Materials
Dexamethasone was provided by Iran Hormone Pharmaceutical Co. (Tehran, Iran). The following items were purchased from Sigma Chemical Company (St. Louis, MO, USA): Aprotinin A, benzethonium chloride, bovine serum albumin, ethylene diamine tetraacetic acid (EDTA), hexadecyl trimethyl ammonium bromide (HTAB), phenylmethylsulfonyl fluoride, Tween® 20, and Tween® 80 (1%). Formalin solution (35%), methanol (99.8%), and glacial acetic acid were supplied by Merck (Darmstadt, Germany). Colonic levels of interleukin (IL)-6, IL-1β, and tumor necrosis factor-alpha (TNF-α) were measured using enzyme-linked immunosorbent assay (ELISA) kits from ZellBio, GmbH, Germany.
3.2. Plant Material and Preparation of the Methanol Extract
In April 2018, A. cepa L. bulbs were locally sourced from Rasht, Guilan Province, northwestern Iran (voucher specimen number: GUMS-Z12). The onion peel extraction was prepared as previously described (10). The extract was stored in a refrigerator until required for use.
3.3. Determinant of the Total Phenol and Flavonoid Content
Total phenolic content (TPC) was characterized using the Folin-Ciocalteu method, with gallic acid serving as the reference standard (11, 12). The total flavonoid content was determined using the aluminum chloride technique, with quercetin serving as the reference standard (13).
3.4. Acute Toxicity Study
An acute toxicity study for ACPME was conducted in accordance with the OECD 425 guidelines, as previously reported (14).
3.5. Animals
Thirty-six male Wistar rats (12 weeks old, 250 ± 20 g) were provided by the Ethics Committee of Guilan University of Medical Sciences (GUMS) Animal House. The animals were housed under a 12-hour light/12-hour dark cycle, with unrestricted access to food and water, and maintained under regulated conditions (relative humidity of 45 - 65%, temperature of 22 ± 24°C). All experimental protocols were authorized by the Ethics Committee of Guilan University of Medical Sciences in accordance with the National Institute of Health Guidance for the Care and Use of Laboratory Animals (Approval No. IR.GUMS.REC.1399.218).
3.6. Experimental Design
Thirty-six animals were randomly divided into six groups (each consisting of six rats) as follows:
Group I (colitis control group): Normal saline was administered intraperitoneally (i.p.) one day before the induction of colitis and then daily thereafter.
Group II (normal group): A cannulation procedure was performed without inducing colitis, and the rats were treated with normal saline i.p.
Group III (dexamethasone-treated group): Rats received dexamethasone (1 mg/kg, i.p.) one day before colitis induction and daily thereafter.
Groups IV–VI (plant extract-treated Groups): Rats received ACPME (50, 100, or 150 mg/kg, i.p.) one day before the induction of colitis and then daily thereafter.
All drugs, including normal saline, dexamethasone, and ACPME (50, 100, and 150 mg/kg), were freshly prepared using distilled water and Tween® 80 (1%). The animals were euthanized by CO₂ asphyxiation three days after colitis induction. The dose ranges for ACPME (15, 16) and dexamethasone (17) were determined based on previous studies.
3.7. Induction of Ulcerative Colitis and Macroscopic Assessment
Colitis was induced as previously described (18). The percentage of body weight loss in the animals was calculated daily throughout the experiment. After euthanizing the animals by CO2 asphyxiation on day 4, the severity of macroscopic colon injury, the surface area of ulcers, and the percentage of necrosis were assessed according to the previously described method (17). The intestinal segments were then divided into four pieces: Three were immediately frozen in liquid nitrogen for biochemical analyses, and the fourth was fixed in a 10% buffered formalin solution for histopathological analysis.
3.8. Histopathological Evaluation and Biochemical Analysis
A qualified pathologist conducted a blind histopathological and microscopic evaluation using a modified scoring system as described previously (19, 20). The extent of neutrophil infiltration into the intestinal mucosa during acetic acid-induced colon injury was assessed by measuring myeloperoxidase (MPO) activity, following an adjusted approach (21). Additionally, the levels of TNF-α, IL-1β, and IL-6 in the colon tissues were quantified using ELISA kits (ZellBio, GmbH, Germany), according to the method outlined by Nacife et al. (22).
3.9. Statistical Analysis
Statistical analysis was performed using SPSS statistical software (Version 17.0). Data are presented as mean ± standard error of the mean (SEM). For parametric data, one-way analysis of variance (ANOVA) was employed to compare the groups, followed by the Tukey post hoc test. Additionally, the Kruskal-Wallis test and post hoc Mann-Whitney U tests were utilized for the statistical analysis of nonparametric data, which are reported as medians. A P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Total Phenol and Flavonoid Content of Allium cepa L. Peel Methanolic Extract
Based on the calibration curve for gallic acid (y = 0.00085x + 0.0398, R² = 0.9981), the total phenolic content of ACPME was determined to be 223.38 ± 0.002 mg of gallic acid equivalents (GAE) per gram of dry extract. Using the standard curve for quercetin (y = 0.0169x - 0.0121, R² = 0.997), the total flavonoid content was calculated to be 49.42 ± 0.008 mg of quercetin equivalents (QE) per gram of extract.
4.2. Lack of Acute Toxicity of Allium cepa L. Peel Methanolic Extract
Following oral administration of the extract at a dose of 2000 mg/kg, all rats survived the 14-day interval of toxicity testing. Additionally, compared to the control group, animals receiving oral ACPME did not exhibit symptoms of toxicity, including behavioral changes, abnormalities in food and water consumption, or weight discrepancies. Consequently, A. cepa L. methanol extract demonstrates an acute oral toxicity (LD50) greater than 2000 mg/kg body weight in Wistar rats.
4.3. Effect of the Allium cepa L. Peel Methanolic Extract on Animal Body Weight and Macroscopic Characteristics
Following 4 days of drug administration, all rats survived throughout the experiment across all groups. As shown in Table 1, animals in the colitis control group exhibited a significant decrease in body weight after 4 days of colitis induction (P < 0.001) compared to those in the normal group. Although a similar pattern was observed in animals receiving ACPME (50 and 100 mg/kg) or dexamethasone, the percentage of weight loss in these groups was markedly lower than in the colitis control group (P < 0.05, P < 0.05, and P < 0.01, respectively).
| Group | Colonic Weight/Length Ratio (mg/cm) | Body Weight Loss After 4 Days (%) | Ulcer Severity (0 - 15) | Total Colitis Index (0 - 10) | Ulcer Area (cm2) | Necrosis (%) |
|---|---|---|---|---|---|---|
| Normal | 95.0 ± 3.6 c | -3.1 ± 0.5 c | 0 | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c |
| Colitis control | 261.6 ± 13.2 | 7.7 ± 0.9 | 12.5 (9 - 14) | 9.3 ± 0.3 | 5.6 ± 0.2 | 46.6 ± 1.5 |
| Dexamethasone | 190.8 ± 5.2 d | 4.5 ± 0.3 d | 4.5 (3 - 7) e | 4.6 ± 0.7 c | 2.8 ± 0.2 c | 17.5 ± 2.1 c |
| ACPME (50 mg/kg) | 190.8 ± 15.8 d | 5.0 ± 0.4 e | 4.5 (4 - 7) e | 5.6 ± 0.6 d | 3.5 ± 0.4 d | 23.1 ± 7.6 d |
| ACPME (100 mg/kg) | 206.6 ± 16.0 e | 5.1 ± 0.4 e | 5 (3 - 7) e | 6.3 ± 0.7 e | 3.7 ± 0.6 e | 26.5 ± 4.5 e |
| ACPME (150 mg/kg) | 243.3 ± 12.8 | 6.4 ± 0.5 | 8.5 (4 - 11) | 7.3 ± 0.6 | 4.2 ± 0.3 | 31.4 ± 4.6 |
Segments derived from the normal group showed no signs of damage to the colon wall. However, severe inflammation, ulceration, hemorrhage, necrosis, and thickening of the colon wall were observed in the colitis control group (P < 0.001). Although inflammatory complications persisted in samples from the treatment groups, there was a significant reduction in the macroscopic characteristics of these groups compared to the colitis control group (Table 1). A substantial decrease in ulcer severity was observed in rats treated with dexamethasone (P < 0.05) and ACPME (P < 0.05 for 50 mg/kg and 100 mg/kg) compared to the colitis control group.
In contrast to the colitis control group, a significant reduction in the ulcer area and the percentage of necrosis was found in animals receiving dexamethasone (P < 0.001) or ACPME (P < 0.01 for 50 mg/kg and P < 0.05 for 100 mg/kg). Furthermore, dexamethasone (P < 0.01) and ACPME (P < 0.01 for 50 mg/kg and P < 0.05 for 100 mg/kg) substantially reduced the colon weight/length ratio compared to the colitis control group.
4.4. Effect of Allium cepa L. Peel Methanolic Extract on Histopathological Characteristics
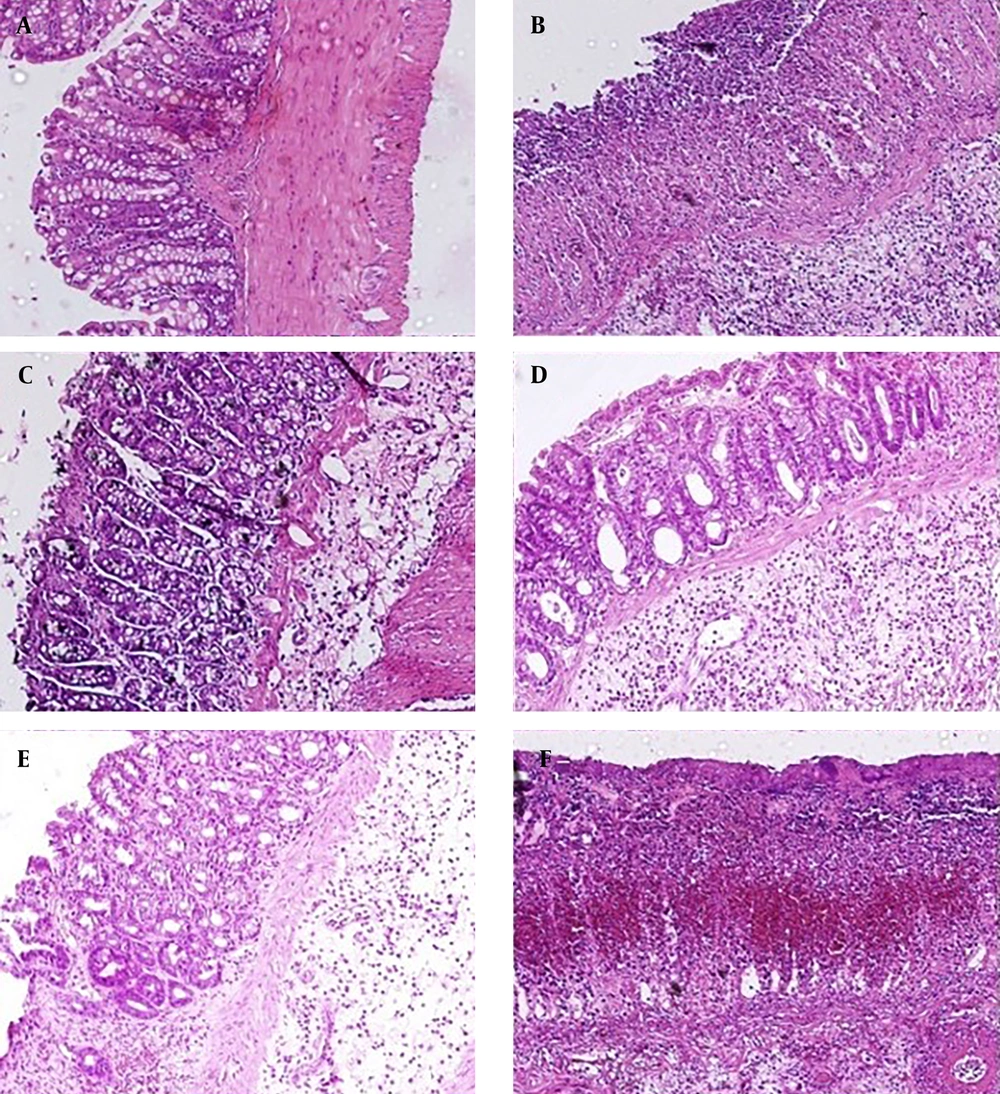
As indicated in Figure 1A - D, histological evaluation of the colon tissues from the normal group revealed normal architecture and unaltered epithelium in the colonic mucosa. In contrast, the colitis control group exhibited significant and excessive transmural inflammation, generalized necrosis, inflammatory granulomas, and submucosal neutrophil infiltration. The total colitis index of the colon segments in the ACPME-treated groups (P < 0.01 for 50 mg/kg and P < 0.05 for 100 mg/kg) and the dexamethasone-treated group (P < 0.001) was significantly lower than that of the colitis control group (Table 1). Additionally, regeneration of the epithelium and decreased inflammatory cell infiltration in the lamina propria were observed in the treatment groups.
Microscopic feature of the colon in experimental colitis (induced by acetic acid) in rats (hematoxylin and eosin staining; original magnification 10×). A, normal group: Mucus layer and crypts are normal; B, acetic acid control group: Epithelial destruction, architectural deformity of the crypts, and inflammatory cell infiltrates; C, dexamethasone (1 mg/kg): Mild to moderate mucosal and submucosal inflammation and mucosal inflammatory cell infiltrates; D and E, the methanol extract of Allium cepa L. peel methanol extract (50 and 100 mg/kg): Mild to moderate mucosal and submucosal inflammation and mucosal inflammatory cell infiltrates; and F, the methanol extract of Allium cepa L. peel methanol extract (150 mg/kg): Destruction of mucosal architecture and infiltration of neutrophils.
4.5. Effect of the Allium cepa L. Peel Methanolic Extract on Myeloperoxidase Activity
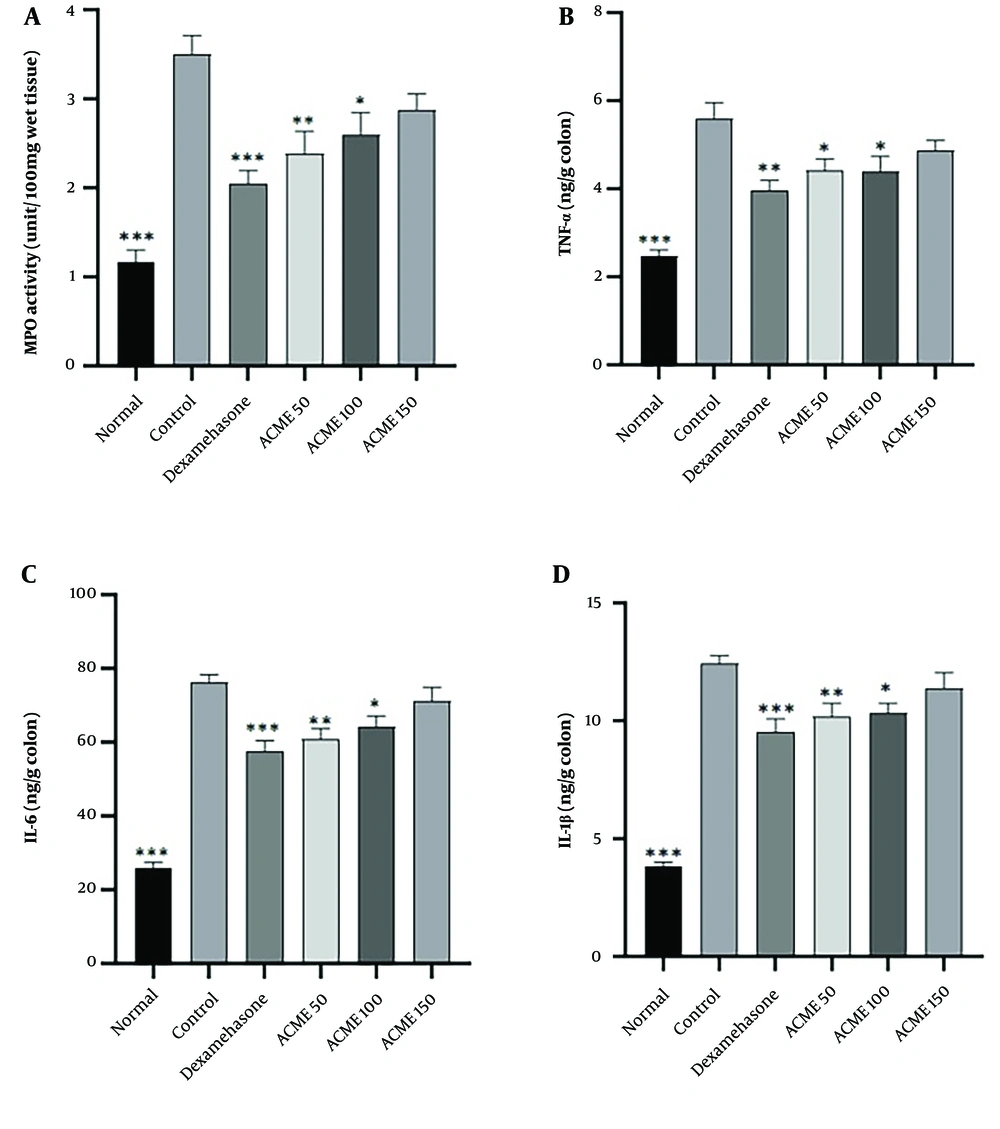
A significant increase in MPO activity was observed in rats treated with acetic acid, as shown in Figure 2A (P < 0.001). Although elevated levels of MPO activity were noted in animals treated with dexamethasone (1 mg/kg) and ACPME (50 and 100 mg/kg), a significant reduction in this parameter was observed in these groups compared to the colitis control group (P < 0.001, P < 0.01, and P < 0.05, respectively).
Effects of the methanol extract of Allium cepa L. peel methanol extract (50, 100, and 150 mg/kg, i.p) or dexamethasone (1 mg/kg, i.p) on biochemical parameters of the rat colon in acetic acid-induced colitis; ACPME = The methanol extract of Allium cepa L. peel methanol extract (50, 100, and 150 mg/kg); IL-6 = interleukin-6; IL-1β = interleukin-1 beta; MPO = myeloperoxidase; TNF-α = tumor necrosis factor-alpha.Values are presented as mean ± SEM, n = 6. A statistical analysis was performed using ANOVA followed by a TUKEY post hoc test. ***P < 0.001, **P < 0.01 and * P < 0.05: Significant difference compared to colitis control group.
4.6. Effect of the Allium cepa L. Peel Methanolic Extract on Cytokine Profile
In rats treated with acetic acid, the levels of pro-inflammatory cytokines in the colon were measured across different treatment groups and compared to the colitis control group. As shown in Figure 2B - D, the colitis control group exhibited significantly increased colonic levels of TNF-α, IL-6, and IL-1β compared to the normal group (P < 0.001). Compared to the colitis control group, the colonic level of TNF-α was significantly reduced in rats treated with dexamethasone (P < 0.01) or ACPME (P < 0.05 for 50 mg/kg and P < 0.05 for 100 mg/kg). Animals treated with ACPME (P < 0.01 for 50 mg/kg and P < 0.05 for 100 mg/kg) or dexamethasone (P < 0.001) showed a significant reduction in the colonic level of IL-6 compared to the colitis control group. Furthermore, the colonic level of IL-1β was markedly decreased in rats receiving dexamethasone (P < 0.001) or ACPME (P < 0.01 for 50 mg/kg and P < 0.05 for 100 mg/kg) compared to the colitis control group (Figure 2D).
5. Discussion
Our research demonstrated that administering the peel methanolic extract of A. cepa significantly alleviated inflammation in acetic acid-induced colitis in rats. The investigation into the oral acute toxicity of ACPME yielded promising results, showing no instances of mortality or adverse effects, suggesting that the median lethal dose (LD50) is greater than 2000 mg/kg in rats. These findings support the potential safe use of ACPME as an additive in traditional applications.
Supported by several evaluations, our results showed that the plant effectively reduced body weight loss percentage, macroscopic and microscopic colon injuries, and inflammatory biomarkers in an experimental model of colitis. Red onion peel is a globally cultivated vegetable with a long history of use in traditional medicine (5, 6). The anti-inflammatory properties of onion peel have been demonstrated through the inhibition of various inflammatory activities, neutrophil infiltration, and oxidative stress in vivo and in vitro (23, 24).
Kang et al. showed that onion peel hot water extract ameliorates lipopolysaccharide-induced inflammatory response by suppressing pro-inflammatory cytokine release and inhibiting cyclooxygenase (COX)-2, inducible nitric oxide synthase (iNOS), nuclear factor-kappa B (NF-κB), and mitogen-activated protein kinase (MAPK) expression in a dose-dependent manner (24). They also reported that onion peel ethanol extract could decrease mouse ear edema and thickness in croton oil-induced mouse ear edema (24). A study by Kim et al. demonstrated the cytotoxic and anti-inflammatory characteristics of onion peel extract in human colon carcinoma cells stimulated by lipopolysaccharides by downregulating TNF-α mRNA expression (25). In another study by Lee et al., the authors reported the anti-inflammatory effects of A. cepa L. peel extracts via inhibition of the Janus kinase signal transducers and transcription activators pathway (JAK-STAT) in RAW264 cells stimulated with lipopolysaccharide (LPS) and reduction in iNOS production, nitric oxide (NO), IL-1α, IL-1β, IL-6, and IL-2 (26).
In line with these studies, our results indicated that ACPME administration decreased MPO activity and levels of IL-6, IL-1β, and TNF-α in inflamed colon tissue. Therefore, we conclude that A. cepa may suppress the synthesis or release of pro-inflammatory cytokines, leading to favorable outcomes in experimental colitis.
The peel of onion is rich in numerous phenolic compounds, including kaempferol, ferulic acid, gallic acid, protocatechuic acid, and quercetin (27). Notably, onion peel contains 20 times more quercetin than the edible flesh (28). Quercetin has demonstrated a broad spectrum of pharmacological characteristics, including anti-inflammatory and antioxidant activities (25, 29, 30). Several studies have shown quercetin's ability to reduce IL-6 (31), IL-1β (32), TNF-α (33), and MPO activity in vitro (34). Quercetin has been confirmed to attenuate pro-inflammatory cytokine production in Porphyromonas gingivalis LPS-treated human gingival fibroblasts through activation of the peroxisome proliferator-activated receptor (PPAR)-γ and suppression of the NF-κB signaling pathway (35). Furthermore, quercetin's anti-inflammatory property in acetic acid-induced colitis was shown to inhibit oxidative stress and reverse the localized inflammatory response (36).
Research by Boots et al. indicated that quercetin exerts a beneficial effect on cellular glutathione (GSH) content, enhancing the capacity to remove free radicals (37). Additionally, two animal models of UC demonstrated that gallic acid had an anti-inflammatory impact, potentially regulated by inhibition of the NF-κB signaling pathway and prevention of p-STAT3Y705 expression (38-40). Based on the studies mentioned above, it appears that the phenolic and flavonoid compounds in ACPME may be at least partly responsible for the anti-inflammatory properties of A. cepa in an animal model of experimental colitis.
The results of this study also illustrated that while the first two doses of ACPME (50 and 100 mg/kg) decreased the severity of colon damage parameters in acetic acid-induced colitis, the highest dose of the extract (150 mg/kg) was less effective in attenuating these parameters. It should be noted that polyphenols and flavonoids not only act as antioxidants but can also behave as pro-oxidants under certain conditions, depending on their concentration (41). Although studies on the pro-oxidant activity of red onion peel have not been reported, its primary compound, quercetin, exhibits pro-oxidant activity at high doses. Meanwhile, the nanomolar concentration of quercetin oxidation products protects against cell oxidative damage (42, 43).
5.1. Conclusions
Overall, our results indicate that the administration of ACPME may have beneficial effects on UC in an experimental model of colitis, potentially by modulating inflammation. This agricultural waste could serve as a source of bioactive molecules advantageous in treating patients with UC. However, further research is necessary to isolate and study the main purified ingredients with anti-inflammatory effects and to elucidate their potential mechanisms. Additionally, further toxicological assessments and evaluations of the oral and chronic administration of red onion peel extract on IBD are recommended, as the route and duration of administration could alter the pharmacokinetics of the active components.