1. Background
Coronavirus disease 2019 (COVID-19) spread rapidly and was declared a 21st-century pandemic. From the onset of the outbreak, intense efforts within the medical community have focused on developing effective diagnostic and treatment methods (1). Although promising results have been reported regarding the effectiveness of various pharmacological agents (2) and COVID-19 vaccinations (3), research continues to focus on identifying low-cost, highly effective, and readily available drugs to mitigate the adverse outcomes of COVID-19 (4). Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA) inhibitors, are primarily used for lipid-lowering but have also been considered as adjuvant immunomodulators against infectious diseases (5). Evidence suggests a reduced risk of pneumonia-related death and a decreased need for intubation among statin users (6).
It is known that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) triggers inflammation through various molecular pathways, which may explain why statin use has been linked to improved COVID-19 outcomes in recent studies (7). For instance, SARS-CoV-2 binding to toll-like receptors (TLRs) initiates the inflammatory cascade via nuclear factor κB (NF-κB) activation, a process that statins are known to inhibit (8). Furthermore, statins may reduce SARS-CoV-2 infectivity by attenuating CD147, a host cell receptor involved in viral entry (9). However, there is concern that statins upregulate angiotensin-converting enzyme 2 (ACE2) receptor expression, which is essential for SARS-CoV-2 invasion of airway epithelial cells (8). While some studies have reported a significantly lower risk of COVID-19 mortality among statin users (10, 11), others have indicated that statin use did not reduce COVID-19 mortality (12) and may even increase the risk of severe infection (13).
2. Objectives
Given these controversial findings and the lack of trials directly supporting the initiation of statins post-SARS-CoV-2 infection with respect to inflammation modulation, we conducted the current study to help address these uncertainties. Despite the formal end of the COVID-19 pandemic, ongoing research to improve COVID-19 outcomes remains relevant. As no specific antiviral treatment exists for COVID-19, exploring affordable and accessible therapeutic options is essential for dealing with future viral epidemics and pandemics.
3. Methods
3.1. Study Design
The double-blind randomized controlled trial (RCT) was conducted on patients diagnosed with confirmed COVID-19 who had been admitted to the hospital. All subjects voluntarily enrolled from June 22 to August 23, 2021, after meeting the inclusion criteria.
3.2. Patients
Newly admitted patients aged over 18 years, with molecular results (qPCR) suggestive of or confirming a diagnosis of SARS-CoV-2 RNA (from 24 hours prior), and exhibiting moderate pulmonary involvement (less than 50%) as evidenced by computed tomography (CT) scans, were screened for enrollment in the trial. Statin users (patients with hypercholesterolemia and/or cardiovascular disease) or those with contraindications to statins, including hypersensitivity or complications after statin use, long-term use of corticosteroids, active liver disease, lung disease, shock, sepsis, pregnancy and breastfeeding, and death, were excluded. Additionally, patients or their families' dissatisfaction with the study, hospitalization in intensive care units (ICU), or requiring invasive ventilation, as well as not completing the follow-up, were grounds for exclusion. All patients provided informed consent to participate in the study before initiating the treatments.
3.3. Ethical Approval
The protocol of this randomized, controlled clinical trial was approved by the Ethics Committee of Shahrekord University of Medical Sciences (IR.SKUMS.REC.1400.046) and registered on www.irct.ir as IRCT20210426051090N1. The clinical trial was conducted in accordance with ethical principles originating from the Declaration of Helsinki and good clinical practice guidelines.
3.4. Randomization and Blinding
The clinical trial, with parallel groups of control and intervention, was conducted on randomly assigned patients (in a 1:1 ratio) to reduce confounding effects. Random allocation software generated a random sequence to support balanced block randomization with block sizes of 4 and 6. Unique codes were assigned to each patient (e.g., code AB1 for patient number one). Envelopes with special numbers for each patient containing nameless tablets related to the intervention or control group were prepared. Allocation was concealed, and only the researcher in charge of the study was aware of the allocation and coding. Physicians, outcome appraisers, and patients in both groups were blinded to the drug content of each envelope.
3.5. Relevant Concomitant Care
After randomization, patients in both groups received remdesivir 200 mg on the first day, followed by 100 mg daily for the next four days.
3.6. Interventions
The intervention group received a daily 40 mg tablet of atorvastatin for 14 days, irrespective of hospital discharge status. The control group received placebo tablets instead of atorvastatin, which were similar in shape, size, color, and duration of use to atorvastatin, and continued for 14 days. Both atorvastatin and placebo tablets were purchased from Dr. Abidi Pharmaceuticals Company, Tehran, Iran, funded by the study. Since Abidi Company did not financially support the study, they did not have access to the study data either during the trial or before publication.
3.7. Outcome Measures
The COVID-19-related outcomes, including clinical signs and symptoms (e.g., body temperature, chills, body pain, sore throat, dyspnea, nausea, vomiting or diarrhea, and headache), oxygen saturation (SpO2), type of O2 supply, laboratory parameters such as coagulation profile, liver function, lipid profile, and surrogate biomarkers of COVID-19-associated inflammation (as the primary outcome), were assessed at the onset of admission and at 7-day intervals. The following formula was used, considering α = 5%, corresponding to a confidence level of 95% and a power (1 – β) of 90%. The sample size was determined to be 84 individuals for both the atorvastatin and placebo groups. Finally, accounting for sample dropouts, a total of 210 patients suffering from moderate COVID-19 infection were included to screen for eligibility.
3.8. Statistical Analysis
According to the Kolmogorov-Smirnov test, quantitative parameters were reported as mean ± standard deviation (SD) for normally distributed data and as median (25th - 75th percentiles) for non-normally distributed data. Qualitative values were reported as frequency (percentage). An independent t-test was used for quantitative variables, and the chi-square test was used for categorical variables to compare the two groups at baseline, on the 7th day, and on the 14th day. Paired t-tests (for two measurements) or analysis of variance (ANOVA) (for repeated measurements) were utilized to compare the beginning to the end of the treatment in each group. The Statistical Package for the Social Sciences (version 24; SPSS Inc., Chicago, IL, USA) was used for data analysis. The significance level was set at P < 0.05.
4. Results
4.1. Demographics and Baseline Characteristics
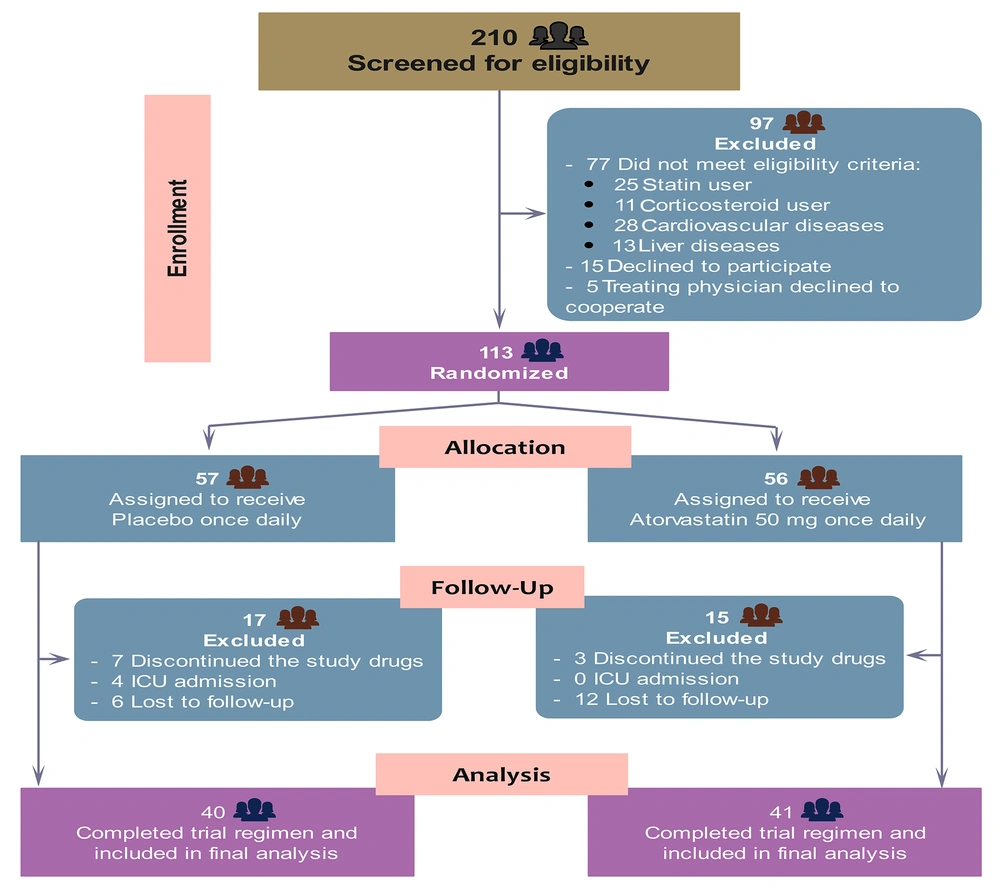
A total of 210 patients were assessed for eligibility, of whom 97 did not meet the inclusion criteria. Finally, 113 patients were enrolled and randomly assigned to receive atorvastatin (n = 56) or placebo (n = 57). After excluding 10 patients who did not receive at least one dose of the study drugs, 18 who were lost to follow-up due to discharge before completing the study drugs, and 4 who were admitted to the ICU requiring mechanical ventilation, 81 patients entered the final analysis population (Figure 1). The demographic, clinical, and laboratory characteristics of the two groups of patients at baseline are shown in Table 1. The two groups were balanced, except for a history of headache, which was more common in the atorvastatin group than in the placebo group (77.5% vs. 55%), and median high-density lipoprotein (HDL), which was significantly lower in patients assigned to atorvastatin than to placebo (32 vs. 36 mg/dL).
| Characteristics | Atorvastatin Group (n = 41) | Placebo Group (n = 40) |
|---|---|---|
| Age (y) | 53.2 ± 13.2 | 53.9 ± 14.7 |
| Gender (male) | 24 (60) | 21 (52.5) |
| BMI | 26.8 (23.3 - 29.4) | 27.1 (24.2 - 32.5) |
| Clinical features | ||
| Chills | 33 (82.5) | 32 (80) |
| Muscular pain | 31 (77.5) | 27 (67.5) |
| Sore throat | 19 (47.5) | 16 (40) |
| Cough | 33 (82.5) | 31 (77.5) |
| Dyspnea | 34 (85) | 34 (85) |
| Nausea and vomiting | 12 (30) | 14 (35) |
| Diarrhea | 6 (15) | 8 (20) |
| Headache b | 31 (77.5) | 22 (55) |
| Body temperature | 37.7 (37.2 - 38.3) | 38.1 (37.1 - 38.5) |
| SpO2 | 88 (85 - 90) | 89 (85 - 91) |
| Laboratory values | ||
| CBC | ||
| WBC (109/L) | 6.687 ± 3.632 | 6.895 ± 4.541 |
| NEU (109/L) | 7.820 (7.0 - 8.640) | 7.995 (7.095 - 8.430) |
| LYM (109/L) | 1.820 (0.875 - 2.543) | 1.635 (1.220 - 2.226) |
| Hb (g/L) | 141 ± 19 | 142 ± 2 |
| PLT (109/L) | 160 (122 - 210) | 156 (125 - 209) |
| Coagulation tests | ||
| PT | 11 (10.3 - 11.4) | 11 (10.2 - 11.5) |
| PTT | 26 (25 - 27.3) | 26 (25 - 27.5) |
| INR | 1.09 ± 0.15 | 1.08 ± 0.1 |
| Liver function tests | ||
| TBil | 0.5 (0.4 - 0.7) | 0.5 (0.4 - 0.8) |
| AST (IU/L) | 41.5 (23.2 - 58.7) | 42.5 (28.5 - 73.5) |
| ALT (IU/L) | 31.5 (18.5 - 47.2) | 24 (20.3 - 57.2) |
| ALKP (IU/L) | 118.5 (95.2 - 188) | 119 (109 - 158.8) |
| Inflammatory biomarkers | ||
| LDH (IU/L) | 280.5 (237.7 - 446.7) | 314.5 (189.5 - 472.3) |
| CRP (mg/L) | 535 (255 - 727) | 405 (153 - 858) |
| ESR (mm/h) | 29 (15.2 - 43.2) | 22.5 (15.3 - 39.2) |
| Ferritin (μg/L) | 47.1 (25.9 - 82.5) | 41.2 (15.8 - 100) |
| IL-6 (ng/L) | 41.44 ± 11.36 | 37.86 ± 12.61 |
| Lipid profile | ||
| Triglyceride (mg/dL) | 82.5 (72 - 121) | 90.5 (66.2 - 115) |
| Cholesterol total (mg/dL) | 135 (106 - 148.7) | 137 (123.8 - 156.7) |
| LDL (mg/dL) | 80 (73 - 100.3) | 80 (62.3 - 100.8) |
| HDL (mg/dL) b | 32 (29 - 37.5) | 36 (29 - 44.8) |
Baseline Characteristics of Enrolled Inpatients a
4.2. Outcomes
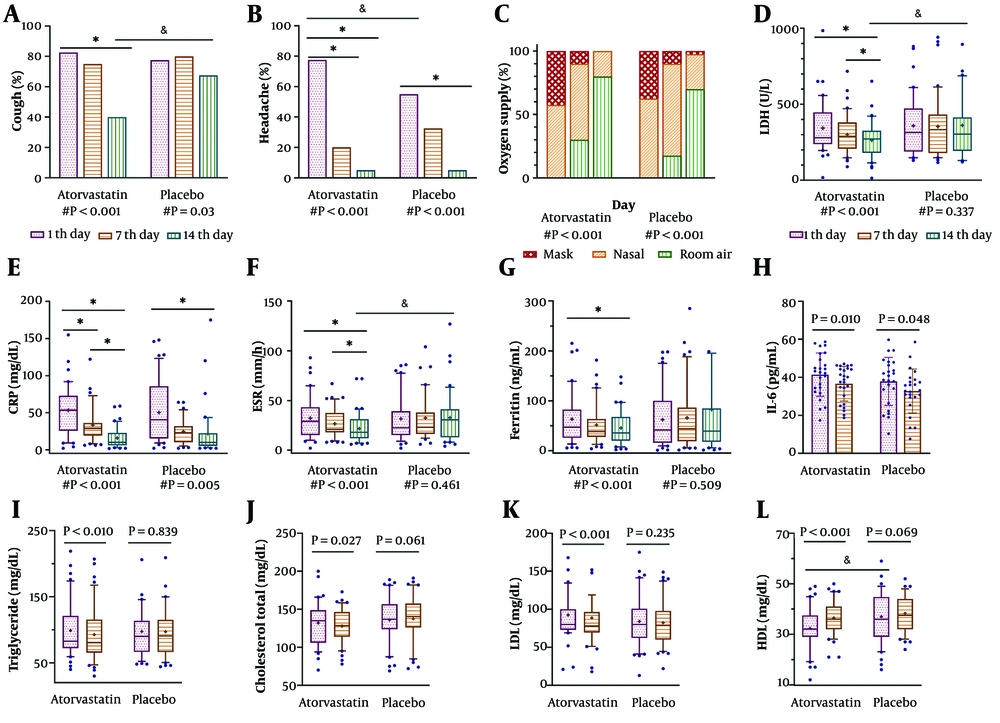
Patients who received atorvastatin were discharged earlier and subsequently lost to follow-up more often than those in the other group (27.5% vs. 10%, P < 0.045); however, no significant relationship was observed between the two groups (r = 0.224, P = 0.103). The aforementioned patients were not included in the final analysis. Our results at the end of the follow-up period showed that all clinical symptoms improved in both groups (Table 2). However, atorvastatin was significantly more effective than placebo in attenuating cough and headache (Figure 2A, B). Figure 2C shows that all patients in both groups at baseline required oxygen supply in the form of nasal or mask ventilation (P = 0.163), but their need decreased significantly by the end of the study in both groups (P < 0.001). No significant difference was seen between the two groups on days 7 or 14 (P = 0.457, P = 0.095, respectively). The results of oxygen saturation also confirm the above findings; the SpO2 median improved from 88% to 94% in the atorvastatin group and from 87% to 92% in the placebo group over the study period (P < 0.001 for both groups). However, there was no significant difference in SpO2 between the two groups at all three measured times (Table 3).
| Variables and Days | Atorvastatin Group (n = 41) | Placebo Group (n = 40) | P-Value b |
|---|---|---|---|
| Chills | |||
| 1 | 33 (82.5) | 32 (95) | 0.775 |
| 7 | 3 (7.5) | 7 (17.5) | 0.176 |
| 14 | 0 (0) | 0 (0) | - |
| P-value c | < 0.001 d | < 0.001 d | |
| Muscular pain | |||
| 1 | 31 (77.5) | 27 (67.5) | 0.317 |
| 7 | 11 (27.5) | 16 (40) | 0.237 |
| 14 | 0 (0) | 1 (2.5) | 0.314 |
| P-value c | < 0.001 d | < 0.001 d | |
| Sore throat | |||
| 1 | 19 (47.5) | 16 (40) | 0.499 |
| 7 | 2 (5) | 1 (2.5) | 0.556 |
| 14 | 0 (0) | 1 (2.5) | 0.314 |
| P-value c | < 0.001 d | < 0.001 d | |
| Cough | |||
| 1 | 33 (82.5) | 31 (77.5) | 0.576 |
| 7 | 30 (75) | 32 (80) | 0.592 |
| 14 | 16 (40) | 27 (67.5) | 0.014 d |
| P-value c | < 0.001 d | 0.030 d | |
| Dyspnea | |||
| 1 | 34 (85) | 34 (85) | 1.00 |
| 7 | 20 (50) | 27 (67.5) | 0.112 |
| 14 | 9 (22.5) | 14 (35) | 0.217 |
| P-value c | < 0.001 d | < 0.001 d | |
| Nausea and vomiting | |||
| 1 | 12 (30) | 14 (35) | 0.633 |
| 7 | 0 (0) | 3 (7.5) | 0.077 |
| 14 | 0 (0) | 0 (0) | - |
| P-value c | < 0.001 d | < 0.001 d | |
| Diarrhea | |||
| 1 | 6 (15) | 8 (20) | 0.556 |
| 7 | 1 (2.5) | 4 (10) | 0.166 |
| 14 | 0 | 1 (2.5) | 0.314 |
| P-value c | 0.006 d | 0.016 d | |
| Abdominal pain | |||
| 1 | 11 (27.5) | 8 (20) | 0.431 |
| 7 | 2 (5) | 2 (5) | 1.00 |
| 14 | 0 | 1 (2.5) | 0.314 |
| P-value c | < 0.001 d | 0.002 d | |
| Headache | |||
| 1 | 31 (77.5) | 22 (55) | 0.033 d |
| 7 | 8 (20) | 13 (32.5) | 0.204 |
| 14 | 2 (5) | 2 (5) | 1.00 |
| P-value c | < 0.001 d | < 0.001 d | |
| Body temperature | |||
| 1 | 37.7 (37.2 - 38.3) | 38.1 (37.1 - 38.5) | 0.451e |
| 7 | 37 (36.8 - 37.1) | 37 (36.9 - 37.3) | 0.08 e |
| 14 | 37 (36.8 - 37) | 37 (36.9 - 37) | 0.284 e |
| P-value c | < 0.001 d | < 0.001 d |
Comparison of Clinical Symptoms Between the Studied Groups in Three Times a
Patients' clinical status and laboratory parameters were improved by atorvastatin. Comparison of cough, headache (A and B), and type of oxygenation (C) between the two groups over the study. Plots display the frequency percentage of variables. The # shows the P-value of comparing the baseline to the end of the study in each group using the Friedman test. Columns were compared pairwise in each group as * P < 0.05 and between the two groups as & P < 0.05, shown as a line, using the Wilcoxon test and Mann-Whitney U-test, respectively. For those parameters measured three times (D - G), a post hoc test, Dunn's multiple comparisons tests, was used after a significant Friedman test (as # noted below each group); columns in each group were compared pairwise: * P < 0.05 shown as a line. Paired t-test (H) and Wilcoxon test (I - L) were used to compare the parameters measured two times in each group shown as a line. The P-value of comparisons between the two groups, separately on the 1st, 7th, and 14th days, displayed as & P < 0.05 using the Mann-Whitney U-test. Plots show the 25th - 75th percentiles in boxes, the median in the center line, the 10th - 90th percentile in whiskers, and means as + in each column, except for (H) where the boxes and whiskers represent mean ± SD, respectively. The P-value less than 0.05 is shown in bold.
| Variables and Days | Atorvastatin Group (n = 41) | Placebo Group (n = 40) | P-Value b |
|---|---|---|---|
| SpO2 (%) | |||
| 1 | 88 (85 - 90) | 89 (85 - 91) | 0.728 |
| 7 | 91 (89 - 92) | 90 (88 - 93) | 0.458 |
| 14 | 94 (93 - 95) | 93 (91 - 95) | 0.074 |
| P-value c | < 0.001 d | < 0.001 d | |
| WBC (109/L) | |||
| 1 | 6.687 ± 3.632 | 6.895 ± 4.541 | 0.822 e |
| 7 | 9.530 ± 3.367 | 10.630 ± 2.874 | 0.120 e |
| P-value f | < 0.001 d | < 0.001 d | |
| NEU (109/L) | |||
| 1 | 8.505 (7.630 - 8.870) | 8.460 (7.758 - 8.908) | 0.776 |
| 7 | 7.820 (7.0 - 8.640) | 7.995 (7.095 - 8.430) | 0.587 |
| P-value g | 0.002 d | < 0.001 d | |
| LYM (109/L) | |||
| 1 | 1.030 (0.690 - 1.870) | 1.125 (0.813 - 1.773) | 0.918 |
| 7 | 1.820 (0.875 - 2.543) | 1.635 (1.220 - 2.226) | 0.918 |
| P-value g | < 0.001 d | < 0.001 d | |
| PLT (109/L) | |||
| 1 | 160 (122 - 210) | 156 (125 – 209) | 0.824 |
| 7 | 213 (159 - 279) | 215.5 (173.5 - 217.3) | 0.609 |
| P-value g | < 0.001 d | < 0.001 d | |
| Hb (g/L) | |||
| 1 | 141 ± 19 | 142 ± 21 | 0.603 e |
| 7 | 138 ± 15 | 140 ± 20 | 0.603 e |
| P-value f | 0.067 | 0.303 | |
| INR | |||
| 1 | 1.09 ± 0.15 | 1.08 ± 0.1 | 0.935 e |
| 7 | 1.15 ± 0.07 | 1.14 ± 0.09 | 0.306 e |
| P-value f | 0.014 d | 0.001 d | |
| TBil | |||
| 1 | 0.5 (0.4 - 0.7) | 0.5 (0.4 - 0.8) | 0.747 |
| 7 | 0.45 (0.4 - 0.5) | 0.4 (0.3 - 0.5) | 0.869 |
| P-value g | < 0.001 d | 0.004 d | |
| AST (IU/L) | |||
| 1 | 41.5 (23.3 - 58.8) | 42.5 (28.5 - 73.5) | 0958 |
| 7 | 36 (28 - 48.8) | 43.5 (28.8 - 73.3) | 0.149 |
| 14 | 35.5 (26 - 51) | 37 (23.5 - 49) | 0.919 |
| P-value c | 0.337 | < 0.001 d | |
| ALT (IU/L) | |||
| 1 | 31.5 (18.5 - 47.3) | 24 (20.3 - 57.2) | 0.485 |
| 7 | 38.5 (22 - 52.5) | 31 (20.3 - 51.8) | 0.338 |
| 14 | 37.5 (25.8 - 56.3) | 29 (21 - 50.3) | 0.178 |
| P-value c | 0.002 d | 0.128 | |
| ALKP (IU/L) | |||
| 1 | 118.5 (95.3 - 188) | 119 (109 - 158.8) | 0.916 |
| 7 | 112 (98.3 - 167) | 113 (104 - 160.3) | 0.919 |
| 14 | 107.5 (101 - 168.5) | 114 (91.5 - 157.8) | 0.969 |
| P-value c | 0.025 d | 0.004 d | |
| LDH (IU/L) | |||
| 1 | 280.5 (237.8 - 446.8) | 314.5 (189.5 - 472.3) | 0.981 |
| 7 | 289.5 (207.3 - 381) | 314.5 (189.5 - 472.3) | 0.363 |
| 14 | 272 (180 - 326) | 314.5 (189.5 - 472.3) | 0.017 d |
| P-value c | < 0.001 d | 0.377 | |
| CRP (mg/L) | |||
| 1 | 535 (255 - 728) | 405 (153 - 858) | 0.522 |
| 7 | 291 (190 - 362) | 233 (101 - 322) | 0.107 |
| 14 | 102 (61 - 229) | 99 (53 - 224) | 0.889 |
| P-value c | < 0.001 d | 0.005 d | |
| ESR (mm/h) | |||
| 1 | 29 (15.2 - 43.2) | 22.5 (15.3 - 39.3) | 0.665 |
| 7 | 21 (18 - 37.5) | 23 (16.3 - 38) | 0.630 |
| 14 | 18 (12 - 31.3) | 30 (13 - 41.3) | 0.019 d |
| P-value c | < 0.001 d | 0.461 | |
| Ferritin (μg/L) | |||
| 1 | 47.1 (25.9 - 82.5) | 41.2 (15.9 - 100) | 0.476 |
| 7 | 38.3 (27.4 - 63.5) | 43.8 (19 - 86.4) | 0.587 |
| 14 | 35.5 (19.9 - 67.7) | 39.1 (17.6 - 84.7) | 0.082 |
| P-value c | < 0.001 d | 0.509 | |
| IL-6 (ng/L) | |||
| 1 | 41.44 ± 11.36 | 37.86 ± 12.61 | 0.301 |
| 7 | 36.63 ± 9.18 | 32.83 ± 11.55 | 0.185 |
| P-value g | 0.010 d | 0.048 d | |
| Triglyceride (mg/dL) | |||
| 1 | 82.5 (72 - 121) | 90 (66.5 - 113.5) | 0.931 |
| 7 | 80.5 (65.3 - 115.3) | 90.5 (66.2 - 115) | 0.665 |
| P-value g | < 0.001 d | 0.839 | |
| Cholesterol total (mg/dL) | |||
| 1 | 135 (106 - 148.8) | 137 (123.8 - 156.8) | 0.620 |
| 7 | 128.5 (114 - 146.5) | 140.5 (126 - 157.5) | 0.059 |
| P-value g | 0.027 d | 0.061 | |
| LDL (mg/dL) | |||
| 1 | 80 (73 - 100.3) | 80 (62.3 - 100.8) | 0.523 |
| 7 | 78 (69.5 - 96.5) | 79 (60.3 - 98) | 0.729 |
| P-value g | < 0.001 d | 0.235 | |
| HDL (mg/dL) | |||
| 1 | 32 (29 - 37.5) | 36 (29 - 44.8) | 0.037 d |
| 7 | 36 (32 - 41) | 37.5 (32 - 44) | 0.346 |
| P-value g | < 0.001 d | 0.069 |
Comparison or Measured Parameters Between the Studied Groups a
Comparing the results of the laboratory parameters (Table 3), we observed that the two groups did not differ significantly on day 7 or day 14, except for LDH and ESR, where the atorvastatin group had a more significant decrease than the placebo group on the 14th day (272 vs. 314.5 U/L, P = 0.017 and 18 vs. 30 mm/h, P = 0.019, respectively, Figure 2). However, when comparing parameters within each group from the 1st to the 14th days, it was noteworthy that atorvastatin was more effective than placebo in reducing inflammatory markers in addition to improving the lipid profile (Table 3). As shown in Figure 2, the decrease in LDH, ESR, and ferritin in the atorvastatin group was significant (from 228.5 to 272 U/L, from 29 to 18 mm/h, and from 47.1 to 3.5 ng/mL, respectively, all P < 0.001), but not in the placebo group. Although C-reactive protein (CRP) and interleukin-6 (IL-6) showed a noticeable decrease at the end of treatment in both groups, the downward trend was more significant in the atorvastatin group (atorvastatin: From 53.5 to 10 mg/dL, P < 0.0001 and from 41.4 to 37.4 ng/mL, P = 0.022, respectively; placebo: From 40.5 to 9.9 mg/dL, P = 0.005 and from 37.9 to 32.8 ng/mL, P = 0.048, respectively).
5. Discussion
This study investigated the effect of atorvastatin on inflammatory outcomes in a hospitalized population with moderate COVID-19 disease. Our findings revealed that atorvastatin treatment was significantly associated with reductions in both inflammatory markers and lipid levels, supporting the growing evidence of statins' protective effects against COVID-19 progression and severity (10, 11). While most COVID-19 studies have focused on the effectiveness of statins on mortality and/or ICU admissions, fewer studies have explored their effects on inflammatory mediators. Retrospective studies have linked prior statin use with lower inpatient mortality, reduced disease severity, and better clinical outcomes in COVID-19 patients (14, 15), though conflicting results also exist (12, 13). Therefore, designing and undertaking prospective RCTs was suggested to confirm the benefits of statin therapy following hospitalization for COVID-19 (16). Soon afterward, a few RCTs showed a link between statin treatment and reduced oxygen requirements and shorter hospital stays (17, 18). However, inconsistencies remain, with some studies showing no significant effect on clinical deterioration, hospital stay length, mechanical ventilation needs, or mortality (19, 20). Contradictory findings also exist, with another RCT reporting increased hospital stays and delayed symptom improvement with atorvastatin (21).
In our study, while we did not specifically investigate mortality and hospitalization, we observed no significant difference in overall clinical symptoms between the two groups, except for improvements in cough and headache in the atorvastatin group, although it did not delay the recovery process. Importantly, statin users recovered faster, were discharged earlier, and required fewer ICU admissions. Overall, although our findings did not indicate strong improved clinical outcomes, focusing on atorvastatin's positive impact on inflammatory status made our study unique. Statins may reduce COVID-19 severity by inhibiting inflammatory pathways. For example, they can attenuate cytokine storms by inhibiting Toll-like receptor 4 (TLR4), suppressing macrophage activity, and down-regulating TLR2 and TLR4 expression, leading to an anti-inflammatory response (22, 23). Our findings aligned with these mechanisms, as we observed significant reductions in all measured inflammatory markers, including LDH, CRP, ESR, ferritin, and IL-6, in patients receiving remdesivir plus atorvastatin. Notably, only CRP and IL-6 levels decreased in the placebo group, and not to the same extent. No evidence of atorvastatin's effect on a set of inflammatory markers in COVID-19 was found, except for two RCTs that presented only a CRP decrease (17, 18), consistent with ours. Additionally, the atorvastatin group showed significantly lower LDH and ESR levels than the placebo group on day 14. These findings could probably be attributed to atorvastatin’s potential anti-inflammatory benefits in COVID-19. Statins could further suppress SARS-CoV-2 infectivity by disrupting cholesterol-rich lipid rafts, which facilitate viral entry via membrane fusion and endocytosis (24). As the most effective cholesterol-lowering agent, atorvastatin was associated with a significant reduction in plasma cholesterol levels among our COVID-19 patients. This may support lipid raft disruption, potentially contributing to the faster recovery of our patients.
Severe COVID-19 is further associated with hypercoagulability and a high incidence of venous thrombosis (25). It was proposed that statins might reduce COVID-19 thrombotic risk (5); however, conflicting evidence has been obtained (19, 26). Our monitoring of coagulation factors (PLT, PT, PTT, and INR) presented no significant association with atorvastatin treatment. Therefore, atorvastatin did not appear to affect the thrombotic state in our COVID-19 patients. Atorvastatin was also well-tolerated, with no elevated liver enzymes, consistent with the low likelihood of statin-related liver injury (27). Interestingly, we observed a downward trend in liver enzymes across all participants, possibly reflecting COVID-19 recovery (28). Methodological differences across studies—such as COVID-19 severity, treatment duration, and sample size—should be considered when comparing findings. Our small sample size (n = 81) may limit the ability to detect significant differences between groups, suggesting the need for larger studies to reduce result heterogeneity. Nevertheless, our study provides the first RCT-based evidence of atorvastatin’s effects on inflammatory markers in COVID-19 patients with moderate severity. Conducting clinical research during the pandemic imposed additional challenges for our healthcare teams, another limitation that underscores the need for more robust RCTs.
5.1. Conclusions
Atorvastatin therapy in hospitalized COVID-19 patients with moderate disease did not affect oxygen need and SpO2 but had a modest effect on clinical symptoms. Nevertheless, atorvastatin significantly reduced inflammatory markers and plasma lipid levels, supporting its safety and potential benefit as part of COVID-19 therapy. Our findings advocate for the repurposing of atorvastatin in COVID-19 management, suggesting further large-scale RCTs to validate and expand upon these findings.