1. Background
Newborns in the neonatal intensive care unit (NICU) are often prescribed multiple medications to treat various health conditions. However, due to the fact that their organs are not fully developed and are functionally immature, elimination of these drugs can be different compared to older children and adults. As a result, newborns may experience higher levels of these medications in their bodies, leading to an increased risk of toxicity or adverse effects (1). This can increase the risk of drug-related problems (DRPs) for newborns. Drug-related problems can refer to any issue related to the use of medication, such as adverse reactions, drug interactions, medication errors, or inappropriate drug therapy. The complexity of managing multiple medications in NICUs can lead to errors in prescribing, administering, or monitoring medications, which can result in harm to the newborn. Drug-related problems are responsible for a significant portion of hospital admissions worldwide, with some estimates indicating that up to 5 - 10% of hospital admissions are due to DRPs. Furthermore, it is believed that more than half of DRPs could have been prevented with proper medication management practices (2). A patient is not using their medication correctly, it can prevent them from experiencing the full benefits of the prescribed treatment (3). Drug-related problems can happen at any point during treatment, such as prescribing, transcribing, dispensing, and patient use. Clinical pharmacists in collaboration with physicians may be can identify and resolve DRPs to ensure safe and effective medication use for patients (4). Different types of drug therapy problems (DTPs) have been proposed and described in various sources. Although there may be slight variations, they all emphasize the importance of evaluating the appropriateness, effectiveness, safety, and patient adherence to medication as initially outlined by Cipolle et al. (5). The Cipolle classification has been frequently used to identify DTPs (6, 7). Cipolle is considered to be one of the most crucial classification systems for DTPs in Iran, and it is widely implemented (8). Drug therapy problems are classified into seven groups in this system based on the nature of the discord that caused them, such as unnecessary drug therapy, additional drug therapy required, drug therapy inefficacy, over-dosage, under-dosage, adverse drug reaction (ADR), and noncompliance. Each of the seven groups represents a different type of problem that can occur, and by categorizing them in this way, healthcare providers can more easily identify the root cause of the issue and work towards a solution (9). It is important to address all categories of symptoms and medical issues in order to prevent negative clinical consequences. Failure to do so can result in temporary symptom worsening, permanent disability, or even death (6, 10). The susceptibility of neonates to DRPs is increased due to their clinical heterogeneity, which is influenced by factors such as gestational age, weight, and postnatal age that determine drug selection. Crucial steps include identifying factors that increase the risk, promptly reviewing medication therapy, and taking corrective actions for identified DTPs in order to minimize harmful outcomes. Additionally, understanding how often DRPs occur in a healthcare system can aid in creating and executing plans to decrease their prevalence and prevent harm to patients.
2. Objectives
Our objective is to examine the frequency of DRPs and determine the associated factors in neonates with sepsis in the NICU.
3. Methods
3.1. Study Design and Population
Between December 1st, 2021, and March 1st, 2022, a prospective observational study was conducted at three NICUs in Iranian children's hospitals. The study included all patients diagnosed with sepsis, hospitalized in the NICUs for more than 24 hours, aged under one month, and prescribed at least one medication. Patients with chronic underlying diseases and congenital malformations were excluded from the study.
Sepsis was defined as infants presenting with clinical symptoms of sepsis and a positive blood culture or exhibiting the following laboratory symptoms: White blood cells (WBC) < 5,000, positive C-reactive protein (CRP), platelets < 150,000, and absolute neutrophil count (ANC) < 1,500 (11), referred to as clinical sepsis.
The data collection form was developed by examining medical charts, physician orders, and nursing reports. Data collected included neonatal-related information, laboratory parameters, and medication data. Daily recording of both prescribed drugs and the occurrence of DRPs was performed throughout the NICU stay.
The neonates were assessed daily for DRPs by a team consisting of one neonatologist, the chief pharmacist, and two clinical pharmacy residents. Two clinical pharmacists independently reviewed and recorded information on DRPs. Only DRPs classified under the Cipolle classification system were considered for the study. As per this system, DRPs are defined as unwanted events or risks that patients may experience during or related to their drug therapy. These problems hinder or delay the patient from attaining the intended therapeutic goals and necessitate professional judgment for resolution (5). Appendix 1 in Supplementary File contains the details of the Cipolle classification system. The sufficiency of the selected dose was assessed using the Neofax® textbook (Thomson Reuters, New York, USA) and the UpToDate® database (Wolters Kluwer, AlphenaandenRijn, NL) as sources of information. An unfavorable event recorded in the literature linked to one of the drugs being administered was regarded as an ADR. A third pharmacist was consulted in the event of disagreement between evaluators. Physicians were targeted for interventions related to adverse events from prescription drugs, while nurses were targeted for interventions related to drug preparation and administration.
3.2. Statistical Analyses
The neonate characteristics and prevalence of DRPs were analyzed using descriptive statistics. Continuous variables were compared using either the Student’s t-test or Mann-Whitney U-test, while categorical variables were analyzed using the χ² or Fisher’s exact test. A P-value less than 0.05 was considered statistically significant.
A multivariate logistic regression analysis was conducted to evaluate the risk factors for DRPs, with odds ratios and 95% confidence intervals (CI) reported. The presence of DRPs was considered the outcome measure, while other variables were considered potential predictors. Bivariate analyses were initially conducted for each predictor variable, followed by a multivariate analysis that included all independent variables to control for potential confounding factors.
SPSS software (v. 22, IBM Corp., USA) was used for all analyses.
4. Results
4.1. Demographic and Clinical Information
During the research period, NICUs admitted 1,161 neonates. Among them, sepsis was culture-positive in 173 (14.9%) neonates, and 116 (9.9%) neonates were diagnosed with clinical sepsis. Seventeen newborns were excluded from the study due to neurological sequelae (n = 7), congenital anomalies (n = 3), and hospitalization for less than 24 hours (n = 7). Consequently, 272 newborns were enrolled in the study.
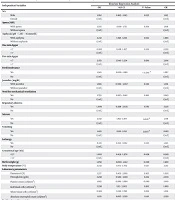
The mean birth weight and gestational weight were 1,781 ± 912 g and 35.4 ± 1.3 weeks, respectively. The average length of NICU stay was 13 days (range: 1 - 142 days). NICU mortality was 9.5%, with 26 deaths recorded. Table 1 provides detailed demographic and clinical data.
| Variables | No. (%) or Mean ± SD |
|---|---|
| Sex | |
| Male | 173 (63.6) |
| Female | 99 (36.4) |
| Apnea (AHI) | 149 (54.7) |
| Asphyxia (pH < 7, BE < -16 mmol/L) | 174 (64.3) |
| One-min Apgar < 7 | 88 (32.3) |
| Five-min Apgar < 7 | 112 (41.1) |
| Birth weight (g) | 1781 ± 912 |
| Body temperature (°C) | 37.8 ± 1.1 |
| Feed intolerance | 193 (70.9) |
| Jaundice (mg/dL) | 96 (35.2) |
| Need for mechanical ventilation | 238 (87.5) |
| Repository distress | 157 (57.7) |
| Seizure | 105 (38.6) |
| Vomiting | 32 (11.7) |
| Lethargy | 34 (12.5) |
| Gestational age (wk) | |
| Preterm ≤ 37 | 140 ± 51.5 |
| Term > 37 | 132 ± 48.5 |
| Laboratory parameters | |
| Hematocrit | 51.01 ± 2.3 |
| Hemoglobin (g/dL) | 18.2 ± 4.1 |
| Platelet count (cell/mm3) | 204636 ± 182021 |
| Red blood cells (cell/mm3) | 4.7 × 106 ± 1.4 × 106 |
| White blood cells (cell/mm3) | 13627.2 ± 1879.1 |
| Absolute neutrophil count (mm3) | 9.47 ± 3.57 |
| Positive C-reactive protein | 89 (32.7) |
| Medication exposure | |
| One antibiotic | 169 (62.1) |
| Two or more antibiotics | 102 (37.5) |
| Antibiotics plus other medications | 172 (63.2) |
| Type of sepsis | |
| Early-onset sepsis | 167 (61.3) |
| Late-onset sepsis | 105 (38.3) |
4.2. Drug-Related Problems
A total of 390 DRPs were identified in the study. Among the 272 neonates with sepsis, 157 (57.5%, 95% CI: 55.8 - 63.8%) experienced DRPs, with an average of 1.4 ± 2.6 DRPs per patient. The majority of drug-related needs were related to effectiveness (171, 43.9%), followed by Safety (169, 43.3%) and indication (50, 12.9%).
According to Cipolle's classification, ineffective drugs were the primary cause of DRPs, accounting for 94 cases (24.1%). The main reasons included the availability of more effective drugs (40 cases, 10.2%) and refractory conditions (33 cases, 8.5%). Another significant factor was excessive dosage (88, 22.6%), particularly when the dosing frequency was too low (37, 9.5%). Table 2 provides further details regarding the reasons behind DRPs.
| Causes of DRP (n = 390) | No. (%) |
|---|---|
| Indication | 50 (12.9) |
| Unnecessary drug therapy | 32 (8.2) |
| No medical indication at this time | 6 (1.5) |
| Duplicate therapy | 10 (2.6) |
| Nondrug therapy more appropriate | 13 (3.3) |
| Treating avoidable adverse reaction | 3 (0.7) |
| Need for additional drug therapy | 18 (4.6) |
| Untreated condition | 12 (3.1) |
| Preventive therapy | 4 (1.0) |
| Synergistic therapy | 2 (0.5) |
| Effectiveness | 171 (43.9) |
| Ineffective drug | 94 (24.1) |
| More effective drug available | 40 (10.2) |
| Condition refractory to drug | 33 (8.5) |
| Contraindication present | 2 (0.5) |
| Inappropriate dosage form | 18 (4.6) |
| Drug not indicated for condition | 1 (0.2) |
| Dosage too low | 77 (19.7) |
| Ineffective dose | 42 (10.8) |
| Drug interaction | 3 (0.8) |
| Duration inappropriate | 19 (4.9) |
| Frequency inappropriate | 13 (3.3) |
| Safety | 169 (43.3) |
| Adverse drug reactions | 81 (20.8) |
| Undesirable effect | 2 (0.5) |
| Drug interaction | 3 (0.7) |
| Incorrect administration | 48 (12.3) |
| Allergic reaction | 6 (1.5) |
| Dosage increase/decrease too fast | 12 (3.1) |
| Unsafe drug for the patient | 10 (2.6) |
| Dosage too high | 88 (22.6) |
| Dose too high | 32 (8.2) |
| Frequency too short | 37 (9.5) |
| Duration too long | 9 (2.3) |
| The dose of the drug was administered too rapidly | 10 (2.6) |
Abbreviation: DRPs, drug-related problems.
During the hospital stay, 318 prescriptions were issued, resulting in a total of 591 medications. Each patient received an average of 2.1 ± 0.4 medicines, and 214 of these contributed to DRPs. Among the drug classes, antimicrobials (131, 61.2%) and respiratory agents (44, 20.6%) were the most involved in DRPs. The highest involvement in DRPs was observed for gentamicin (46, 21.5%) and amikacin (37, 17.3%). Table 3 provides details on the five medications most frequently associated with DRPs.
| Medicines | Medicines Involve in DRP (n = 214) | Causes of DRP | |||||
|---|---|---|---|---|---|---|---|
| Ineffective Drug | Dosage Too Low | Adverse Drug Reactions | Dosage Too High | Unnecessary Drug Therapy | Need for Additional Drug Therapy | ||
| Gentamicin | 46 (21.5) | 13 (6.1) | 23 (10.7) | - | 10 (4.7) | - | - |
| Amikacin | 37 (17.3) | 16 (7.5) | 13 (6.1) | - | 6 (2.8) | - | 2 (0.9) |
| Ampicillin | 24 (11.2) | 4 (1.9) | 3 (1.4) | 12 (5.6) | - | 5 (2.3) | - |
| Vancomycin | 17 (7.9) | - | 8 (3.7) | 3 (1.4) | 2 (0.9) | 1 (0.4) | 3 (1.4) |
| Phenobarbital | 13 (6.1) | - | 7 (3.2) | 3 (1.4) | - | 3 (1.4) | - |
Abbreviation: DRPs, drug-related problems.
a Values are expressed as No. (%).
Pharmacotherapy recommendations were provided for 314 (80.5%) of the identified DRPs. Of these, 192 cases were related to physicians, and 122 cases were related to nurses. The physicians accepted the DRP recommendations in 74.6% of cases, while the nurses accepted them in 80.6% of cases.
4.3. Risk Factors for Drug-Related Problems
The bivariate logistic regression analyses presented in Table 4 demonstrated that the risk of DRPs increased significantly with feeding intolerance and vomiting. Additionally, the risk of DRPs was higher for patients who received multiple antibiotics or combinations of antibiotics with other medications.
| Independent Variables | Bivariate Regression Analysis | Multivariate Regression Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P - Value | OR | 95% CI | P-Value | |
| Sex | ||||||
| Male | 1.262 | 0.883 - 2.165 | 0.232 | 1.183 | 0.785 - 1.751 | 0.432 |
| Female | 1 (ref.) | 1 (ref.) | ||||
| Apnea (AHI) | ||||||
| With apnea | 1.202 | 0.816 - 1.751 | 0.304 | 1.618 | 1.081 - 2.740 | 0.212 |
| Without apnea | 1 (ref.) | 1 (ref.) | ||||
| Asphyxia (pH < 7, BE < -16 mmol/L) | ||||||
| With asphyxia | 2.232 | 1.398 - 3.195 | 0.523 | 1.998 | 1.247 - 2.997 | 0.121 |
| Without asphyxia | 1 (ref.) | 1 (ref.) | ||||
| One-min Apgar | ||||||
| < 7 | 0.995 | 0.418 - 1.827 | 0.124 | 2.230 | 1.863 - 2.820 | 0.541 |
| > 7 | 1 (ref.) | 1 (ref.) | ||||
| Five-min Apgar | ||||||
| < 7 | 2.651 | 1.540 - 3.224 | 0.081 | 2.891 | 1.239 - 3.498 | 0.109 |
| > 7 | 1 (ref.) | 1 (ref.) | ||||
| Feed intolerance | ||||||
| Yes | 1.545 | 0.809 - 1.989 | < 0.001 a | 1.667 | 0.815 - 1.987 | 0.003 a |
| No | 1 (ref.) | 1 (ref.) | ||||
| Jaundice (mg/dL) | ||||||
| With jaundice | 1.846 | 0.990 - 3.067 | 0.594 | 1.194 | 0.619 - 2.322 | 0.763 |
| Without jaundice | 1 (ref.) | 1 (ref.) | ||||
| Need for mechanical ventilation | ||||||
| Yes | 1.759 | 0.905 - 2.612 | 0.162 | 2.683 | 1.304 - 4.513 | 0.223 |
| No | 1 (ref.) | 1 (ref.) | ||||
| Repository distress | ||||||
| Yes | 1.009 | 0.498 - 2.023 | 0.765 | 2.120 | 1.004-3.129 | 0.342 |
| No | 1 (ref.) | 1 (ref.) | ||||
| Seizure | ||||||
| Yes | 2.012 | 1.192 - 3.107 | 0.022 a | 1.718 | 0.891 - 2.930 | 0.182 |
| No | 1 (ref.) | 1 (ref.) | ||||
| Vomiting | ||||||
| Yes | 1.401 | 0.811 - 2.152 | 0.007 a | 0.819 | 0.389 - 1.980 | < 0.001 a |
| No | 1 (ref.) | 1 (ref.) | ||||
| Lethargy | ||||||
| Yes | 0.513 | 0.323 - 0.912 | 0.132 | 1.223 | 0.792 - 2.982 | 0.221 |
| No | 1 (ref.) | 1 (ref.) | ||||
| Gestational age (wk) | ||||||
| ≤ 37 | 1.002 | 0.439 - 1.970 | 0.098 | 0.985 | 0.321 - 1.972 | 0.321 |
| > 37 | 1 (ref.) | 1 (ref.) | ||||
| Birth weight (g) | 1.056 | 0.690 - 1.242 | 0.099 | 1.980 | 0.965 - 2.213 | 0.650 |
| Body temperature (°C) | 2.342 | 1.874 - 3.762 | 0.127 | 2.212 | 0.989 - 3.214 | 0.901 |
| Laboratory parameters | ||||||
| Hematocrit (%) | 1.237 | 0.453 - 2.893 | 0.103 | 1.003 | 0.322 - 1.900 | 0.224 |
| Hemoglobin (g/dL) | 2.298 | 0.980 - 3.001 | 0.234 | 2.001 | 1.201 - 2.998 | 0.109 |
| Platelet count (cell/mm3) | 1.982 | 0.889 - 2.890 | 0.080 | 2.109 | 1.008 - 3.108 | 0.103 |
| Red blood cells (cell/mm3) | 2.298 | 1.112 - 3.002 | 0.981 | 1.980 | 0.871 - 2.710 | 0.227 |
| White blood cells (cell/mm3) | 0.983 | 0.128 - 1.780 | 0.218 | 1.238 | 0.453 - 2.879 | 0.114 |
| Absolute neutrophil count (cell/mm3) | 1.021 | 0.807 - 2.229 | 0.118 | 2.229 | 1.890 - 3.140 | 0.221 |
| Positive C-reactive protein | ||||||
| Positive | 1.430 | 0.239 - 2.018 | 0.889 | 1.001 | 0.428 - 1.937 | 0.287 |
| Negative | 1 (ref.) | 1 (ref.) | ||||
| Medication exposure | ||||||
| Antibiotic exposure | ||||||
| One antibiotic | 1 (ref.) | 1 (ref.) | ||||
| Two or more antibiotics | 1.239 | 0.650 - 2.972 | 0.005 a | 1.672 | 0.980 - 2.001 | 0.002 a |
| Antibiotics plus other medications | ||||||
| Yes | 2.256 | 1.112 - 3.189 | 0.004 a | 1.118 | 0.895 - 1.998 | 0.009 a |
| No | 1 (ref.) | 1 (ref.) | ||||
| Type of sepsis | ||||||
| Early-onset sepsis | 0.873 | 0.239 - 1.982 | 0.098 | 1.219 | 0.435 - 2.290 | 0.115 |
| Late-onset sepsis | 1 (ref.) | 1 (ref.) | ||||
a P-value < 0.05.
While laboratory parameters and the type of sepsis [early-onset sepsis (EOS) or late-onset sepsis (LOS)] did not directly influence the risk of DRPs, DRPs were found to be more common among EOS patients (OR: 1.342, 95% CI: 1.175 - 1.524, P < 0.001).
To control for confounding variables, a multivariate logistic regression analysis was performed. The results, also shown in Table 4, confirmed the continued statistical significance of feeding intolerance, vomiting, and drug count as independent risk factors for DRPs.
5. Discussion
At least 50% of neonates with sepsis were affected by DRPs in this study. Our findings revealed a higher DRP rate (57.7%) compared to similar studies conducted in Ethiopia (48.8%) (7) and Brazil (32% and 53%) (1, 12). This variability may be attributed to differences in hospital environments, drug utilization practices, healthcare systems, DRP classification methods, availability of skilled prescribers and pharmacists in NICU wards, and variations in management protocols across countries and over time.
In our study, the most common cause of DRPs was drug ineffectiveness, which accounted for 24.1% of cases. This was frequently due to the use of inappropriate medications for the patient’s condition, emphasizing the need for medication changes to effectively address the issue. One pharmacotherapy recommendation involved switching from gentamicin to tazocin for cases of early sepsis unresponsive to treatment. On the other hand, studies have shown that only 5 out of every 100 neonates given antibiotics upon NICU admission have a positive blood culture (13). Additionally, approximately 35% of newborns in the NICU receive at least one inappropriate antibiotic (14). The prevalence of ineffective drugs as a DRP category in our study aligns with these findings.
According to Nunes et al. (1), drug ineffectiveness was related to 84.8% of DRPs in their study. Research has shown that medication errors in NICUs occur significantly more often compared to hospital settings for adults, with estimates ranging from 3 to 91 medication errors per 100 admissions (15-17). Most NICU medication errors happen during the prescribing phase (18). Additionally, dosage errors have been reported in 42% to 51.5% of cases in NICUs. In our study, 42.3% of DRPs were related to dosage issues, with 19.7% attributed to low dosage and 22.6% to high dosage (15-17, 19).
Selecting appropriate dosages and intervals for neonates is challenging due to their underdeveloped drug absorption, metabolism, and excretion systems. Neonatal medications must be prescribed based on birth weight, gestational age, and postnatal age, with dosages calculated by weight. Failure to perform regular checkups and monitor daily weight changes in newborns often leads to dosage errors. Our study identified a higher percentage of high-dose drug usage (22.6%) compared to studies in Ethiopia (10.9%) (20) and Hong Kong (19.3%) (21). Conversely, the use of low-dose drugs in our study (19.7%) was lower than reported in Ethiopia (27.5%) (20), Egypt (21%) (22), and Saudi Arabia (58.6%) (23). These findings underscore that neonates and children are particularly susceptible to receiving inappropriate medication dosages.
We observed substantial fluctuations in body weight within a few days during the therapeutic follow-up period, highlighting the critical need for ongoing dose adjustments to prevent medication errors.
In this study, incorrect administration was identified as the primary cause of ADRs. For instance, one case involved the administration of norepinephrine via an incorrect route. Nurses were most commonly implicated in these types of DRPs, with the illegibility of physician handwriting being a significant contributing factor. Numerous studies have demonstrated that medication errors frequently arise from challenges in interpreting unclear physician orders (24-27).
To mitigate these issues, technological solutions such as computerized physician order entry (CPOE) and clinical decision support systems (CDSS) have been shown to be effective. Computerized physician order entry enables healthcare providers to enter medication orders and other clinical instructions electronically, reducing the risk of errors caused by poor handwriting. Clinical decision support systems offer evidence-based guidelines and recommendations to healthcare providers, improving patient care and minimizing the likelihood of errors.
Additionally, the implementation of barcodes can enhance medication safety by verifying patient identity and ensuring that the correct medication is administered to the correct patient at the right time. Personal digital assistants (PDAs) further improve communication and access to patient information, reducing reliance on handwritten notes and orders (28-31). These tools collectively address critical vulnerabilities in the medication process, leading to safer and more efficient healthcare delivery.
According to previous studies, the antimicrobial class is the primary class associated with DRPs in neonates (1, 12). In our study, aminoglycosides, along with other antimicrobial drugs, were most commonly involved in DRP occurrences. The main medications associated with DRPs were gentamicin and amikacin, with issues primarily related to "ineffective drugs" and "dosage problems."
Aminoglycosides possess unique pharmacokinetics, such as an increased volume of distribution and renal elimination, which contribute to these challenges (18). Antibiotic dosages for newborns require periodic adjustment to account for changes in weight and gestational age. The complexity of prescribing aminoglycosides for neonates stems from the existence of multiple recommended dose regimens, making it difficult to standardize treatment (32).
Examples of pharmacotherapy recommendations from our study included:
- "Resistance to gentamicin is high in this setting; amikacin or tazocin must be used."
- "Amikacin every 24 hours is too short to produce the desired response; it must be changed to every 36 hours."
Our study identified an unnecessary drug therapy rate of 8.2%, which is comparable to rates reported in previous studies, such as 7.3% in Ethiopia (20) and 3.8% in Saudi Arabia (23). The unnecessary use of drugs poses significant problems, including the development of antibiotic resistance and increased healthcare costs.
To address this issue, various strategies and interventions have been implemented to reduce unnecessary antibiotic exposure. For example, some studies focused on limiting the initiation of antibiotics through targeted interventions (33-35). Others employed automatic stop orders or alerts designed to reduce the duration of antibiotic use (36-39).
Additionally, organizations offering antimicrobial stewardship program (ASP) services have implemented actions such as automatic stop orders and alerts to further shorten the duration of antibiotic therapy (40-42). These approaches highlight the importance of structured interventions and proactive management in minimizing unnecessary drug use and improving overall patient outcomes.
According to the 2016 surviving sepsis campaign (SSC) guideline, empiric combination therapy is recommended for the initial treatment of sepsis (43), a point also emphasized by ASP (44). Combination therapy, which involves using two different antibiotic classes, can effectively target a single pathogen (45). Multiple studies have demonstrated that combination therapy is superior to monotherapy in the treatment of sepsis (46-49).
In our study, only 37.5% of antibiotic therapies involved combination therapy. Additionally, 4.6% of DRPs required additional drug therapy. Comparatively, Awoke et al. (7) found that all treatments utilized combination antibiotic therapy, with 33.5% of patients requiring extra antibiotics, while Leopoldino et al. (12) reported this need in only 3.1% of cases.
Our multivariate analysis revealed that exposure to two or more antibiotics and taking antibiotics alongside other medications increased the likelihood of DRPs. Existing evidence supports this finding, as complex drug regimens are associated with a higher risk of DRPs (50). The increased risk may be due to factors such as drug-drug interactions (51), medication errors (52), or nursing errors (53, 54). Studies consistently show that the risk of DRPs increases with the number of medications taken (7).
A key strength of this study is its comprehensive examination of all factors influencing DRP occurrence. Unlike prior studies conducted at single centers, this research was conducted across multiple NICUs, enhancing the generalizability of the findings. However, the study did not evaluate the severity of DRPs, which can range from mild to severe. Additionally, CDSS integrated with CPOE and barcode dispensing and administration systems—proven to reduce DRPs—were not utilized in our study.
Future research should address the lack of intervention and follow-up in this study to assess DRP outcomes. These limitations underscore the need for more comprehensive approaches to prevent and manage DRPs effectively.
5.1. Conclusions
In the NICU, DRPs are prevalent, often resulting in ineffective drug therapy due to inappropriate dosages and ineffective drug selection. The risk of DRPs is heightened by factors such as feeding intolerance, vomiting, exposure to multiple antibiotics, and the combined use of antibiotics with other medications.
To address this issue, it is essential to develop and implement effective interventions aimed at reducing DRPs. By identifying and managing all potential risk factors, including conditions that are not the primary reason for admission, we can significantly decrease the incidence of DRPs in neonatal sepsis.
Collaboration with clinical pharmacists is critical in this effort. Neonatal care teams can work alongside clinical pharmacists to prevent, detect, and mitigate DRPs. Clinical pharmacists play a pivotal role in the early identification of DRPs and in providing targeted preventive measures, which are vital for minimizing the occurrence of overt DRPs and improving neonatal care outcomes.