1. Background
Acute renal failure (ARF) serves as a model for renal ischemia-reperfusion (RIR), a critical issue in kidney transplantation and a non-specific factor contributing to allograft dysfunction. In transplanted organs, ischemia is categorized into two types: Warm ischemia, which occurs during organ resection and anastomosis, and cold ischemia, which takes place during organ preservation in protective solutions (1-3). In animal studies, warm ischemia is induced by unilateral or bilateral renal pedicle occlusion (4), leading to a temporary disruption of oxygen and nutrient supply to the kidney. This condition triggers cellular and molecular responses, including renal epithelial cell destruction, reduced expression of the mammalian target of rapamycin (mTOR), and increased expression of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and cleaved caspase-3 (cl-caspase-3). These changes contribute to kidney dysfunction and elevated urea and creatinine (Cr) levels (3, 5). During reperfusion, reactive oxygen species (ROS) are generated in the kidney, leading to oxidative damage (6). While inflammation has been identified as a major factor in ischemia-reperfusion injury, treatment options aimed at reducing inflammation remain limited (4, 7, 8). Antioxidants that enhance the antioxidant system and possess anti-inflammatory properties may neutralize ROS during IR, thereby reducing oxidative stress (9, 10).
The phenolic structure of essential oils serves as a key antioxidant and antimicrobial component. Essential oils derived from various traditional plants, including lemon, clove, cinnamon, and thyme, exhibit significant antimicrobial and antioxidant properties, which enhance the durability and quality of food products (11). Thyme essential oil (TEO) has demonstrated potent inhibitory effects against bacterial pathogens such as Staphylococcus aureus and Escherichia coli. Additionally, as a natural antioxidant, TEO serves as an excellent alternative to synthetic compounds for ensuring food safety and quality (12). Beyond its antimicrobial properties, thyme has been reported to improve kidney, spleen, and liver function by reducing inflammation, oxidative stress, and the expression of proinflammatory cytokines (13, 14).
2. Objectives
This study aimed to evaluate the effects of TEO on renal function improvement, oxidative stress reduction, and suppression of proinflammatory cytokines and caspase-3 gene expression following RIR.
3. Methods
3.1. Isolation of Thyme Oil
To isolate the essential oil, 50 g of the whole dried parts of the thyme herb were subjected to hydro-distillation with 250 mL of distilled water using a Clevenger apparatus for 2 hours. The extracted oil samples were dried over anhydrous sodium sulfate, filtered, and stored in a sealed vial at 4°C (15, 16).
3.2. Animals
Thirty-two Wistar Rattus norvegicus rats, weighing 200 - 220 g, were obtained from the Experimental Animal Center of Dezful University of Medical Sciences (Iran). The animals were housed in a pathogen-free environment at a room temperature of 24 ± 2°C and humidity of 50 ± 10%, with a 12-hour light/dark cycle. The research was conducted with the approval of and in accordance with the guidelines of the Animal Care and Ethics Committee of Dezful University of Medical Sciences, Dezful, Iran (IR.DUMS.REC.1395.5).
3.3. Experimental Group
The animals were randomly divided into four groups of eight. Group 1: The sham group, in which the animals underwent laparotomy without RIR surgery; Group 2: The IR group, which underwent 45 minutes of ischemia followed by 24 hours of reperfusion and received 0.5 mL/kg of water administered intraperitoneally daily for one week without any treatment; Group 3: The IR+TEO group, which underwent RIR and was treated with TEO (0.5 mL/kg) administered intraperitoneally daily for one week (17, 18); and Group 4: The TEO group, which did not undergo RIR but received TEO (0.5 mL/kg) administered intraperitoneally daily for one week. Before the procedure, Groups 3 and 4 received thyme oil (0.5 mL/kg), while Group 1 received distilled water for one week as a pretreatment (Figure 1).
3.4. Surgical Procedure
After one week of pretreatment, the animals were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and xylazine (8 mg/kg). A midline incision was made from the xiphoid process to the pubic symphysis to expose the abdominal cavity. The kidneys were identified, and the renal pedicles were isolated. A warm renal ischemia model was established by clamping the renal vessels bilaterally for 45 minutes, during which the kidneys turned pale. Throughout this period, the core body temperature was maintained at 37°C. After 45 minutes, the clamps were removed, and blood reperfusion was confirmed by observing the return of the kidneys to their natural color. Reperfusion was allowed to continue for 24 hours, after which the incision was sutured using 3/0 nylon.
For 24 hours postoperatively, the rats were placed in metabolic cages for urine sample collection. Subsequently, they were anesthetized, and blood samples were collected via cardiac puncture. Both kidneys were harvested; the right kidney was fixed in 10% formalin for histopathological evaluation, while the left kidney was frozen at -70°C for biochemical parameter analysis and gene expression assessment.
3.5. Tissue Preparation
The rats were sacrificed under deep anesthesia, and the right kidney was harvested for histological and stereological analysis. The kidney was preserved in 10% formalin for fixation. Tissue processing was carried out using a graded series of ethanol (70%, 80%, 96%, 96%, and 100%) for dehydration, followed by xylene for clearing. The tissue was then embedded in paraffin, and paraffin blocks were prepared. Sections of 5-µm thickness were obtained using a microtome and stained with hematoxylin and eosin (H&E) and periodic acid–Schiff (PAS) for histopathological evaluation.
3.6. Histological Analysis
These sections were assessed using an Axiovision 40 scale at 40× magnification to evaluate the degree of tubular necrosis and leukocyte infiltration, following a previously established scoring system (19). The scoring criteria were as follows:
0 = No injury; 1 = Damage to a single cell; 2 = Damage affecting < 25% of the tissue; 3 = Damage affecting 25 - 50% of the tissue; 4 = Damage affecting > 50% of the tissue
3.7. Stereological Method
3.7.1. Sampling of the Kidney
To assess the volume density of kidney structures, isotropic uniform random (IUR) sectioning was employed. In this approach, the entire kidney was positioned within a circular template, and a random number was selected to determine the first cutting direction. The remaining portion of the kidney was then placed in a separate circle, where another randomly chosen number guided the second cut. The slab thickness was adjusted to ensure the collection of 8 to 12 uniform sections.
3.7.2. Estimation of the Volume Density
The stereological analysis was conducted using a Nikon E-200 microscope (Nikon, Japan) connected to a computer. Images of each kidney tissue section were examined at 400x magnification. The point-counting technique was employed by overlaying a stereological probe (point grid) onto the displayed images. A systematic random sampling approach was applied, ensuring equal spacing along the x- and y-axes across all histological slides.
To determine the volume density of the proximal convoluted tubules, the point-counting method was utilized. Tubules exhibiting a brush border that intersected the right and upper points of the probe were identified and recorded for analysis. The volume density of the proximal convoluted tubule was calculated using the following formula: ∑ Pp/∑ Pt; ∑ Pp = the sum of points hitting the proximal convoluted tubule epithelium; ∑ Pt = the total number of points in the probe (20)
Similarly, glomerular volume was estimated using the point-counting method. Glomeruli that intersected the right and upper points of the probe were counted. The glomerular volume was estimated using the formula: ∑ Pp/∑ Pt; ∑ Pp; ∑ Pp = the sum of points hitting the glomeruli; ∑ Pt = all points placed on the probe (20)
Additionally, mesangium volume was estimated following the point-counting method. Mesangial structures that intersected the right and upper points of the probe were counted. The mesangium volume was estimated using the same formula: ∑ Pp/∑ Pt; ∑ Pp, ∑ Pp = the sum of points hitting the mesangium; ∑ Pt = all points placed on the probe (21)
These stereological estimations provided an accurate and unbiased measurement of renal structural changes (21) (Figure 2).
3.8. Measurement of Serum Parameters
Twenty-four hours after IR, blood samples were collected from the right ventricle of the heart, and serum was separated by centrifugation at 3000 rpm for 10 minutes. The urea and creatinine levels in the blood, as well as urine creatinine (to assess creatinine clearance), were measured using a Pars Azmon kit and an autoanalyzer (Biotecnica Instruments, BT1000).
Creatinine clearance = urine creatinine × urine flow/serum creatinine (10)
3.9. Measurement of Biochemical Parameters
3.9.1. Measurement of Malondialdehyde
Renal malondialdehyde (MDA) was measured using the thiobarbituric acid method. Absorbance was determined using a spectrophotometer at 532 nm (22).
3.9.2. Measurement of Glutathione
The amount of renal glutathione (GSH) was measured using the method described by Sedlak (23). For this purpose, 100 μL of kidney tissue supernatants were mixed with 500 μL of sulfhydryl reagent 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB) at pH 8.0 and then measured at 412 nm using an ELISA reader.
3.9.3. Measurement of Glutathione Peroxidase
Glutathione peroxidase (GPX) activity in the kidney was measured using the Rotruck method. A mixture of 200 μL of tert-butyl hydroperoxide, 25 μL of homogenized kidney sample, and 100 μL of hydrogen peroxide (H2O2) was prepared and measured at 420 nm using an ELISA reader (24).
3.9.4. Measurement of Catalase
Renal catalase activity was measured using the Aebi method (25). For this purpose, 100 μL of kidney tissue was mixed with 0.8 mL of phosphate buffer, followed by the addition of 10 μL of hydrogen peroxide (H2O2). The absorbance was then measured at 240 nm using a spectrophotometer.
3.9.5. Measurement of Paraoxonase 1
Paraoxonase 1 (PON1) activity was measured using the Blatter method (26). Briefly, paraoxon was used as a substrate, and after homogenization of the kidney tissue, its absorbance was measured at 412 nm using a spectrophotometer.
3.9.6. Measurement of Nitric Oxide
Renal nitric oxide (NO) was measured based on a previous study’s method (24).
3.9.7. Measurement of Myeloperoxidase
A mixture containing phosphate buffer (pH = 6.0), hydrogen peroxide, O-dianisidine, and the sample was placed in a cuvette with a 1 cm path length. The final volume of the mixture was 3 mL. In the final stage of preparation, the sample was added, and changes in absorbance at 460 nm were measured over 10 minutes (27).
3.10. Real-time Quantitative PCR for Oxidative Stress Genes
3.10.1. RNA Extraction
The kidney tissues were homogenized and centrifuged, and total RNA was extracted according to the manufacturer's instructions (AnaCell, Iran). RNA concentration was determined using NanoDrop™ 1000 at 260/280 nm.
3.10.2 Fabrication of cDNA
First, total RNA was reverse-transcribed into cDNA according to the manufacturer’s instructions [Yekta Tajhiz Azma, Iran (YTA)]. For each 2 µL of cDNA, 0.5 µL of forward primer, 0.5 µL of reverse primer, 0.5 µL of SYBR Green, and 5 µL of distilled water were used.
3.10.3 Real-time PCR
To measure gene expression, PCR was conducted at 95°C for 3 minutes, followed by 45 cycles at 95°C for 5 seconds and 60°C for 1 minute. The program was followed by melting curve analysis with linear heating from 60 to 90°C (28).
This analysis was performed using Step One software to measure TNF-α, IL-6, and caspase-3 mRNA expression in renal tissue samples. The mRNA levels for each gene were normalized using β-actin as the housekeeping gene. Data comparisons between experimental groups were conducted in triplicate. Gene sequences were obtained from the NCBI website, and forward and reverse primers were designed using Gen Runner and Primer Express software (Table 1).
| Genes | Primer Sequences (5′-3′) | Length (bp) |
|---|---|---|
| IL-6 | 74 | |
| Forward | CGAAAGTCAACTCCATCTGCC | |
| Reverse | GGCAACTGGCTGGAAGTCTCT | |
| TNF-α | 87 | |
| Forward | CCAGGAGAAAGTCAGCCTCCT | |
| Reverse | TCATACCAGGGCTTGAGCTC | |
| Caspases-3 | 393 | |
| Forward | GGACAGCAGTTACAAAATGGATTA | |
| Reverse | CGGCAGGCCTGAATGATGAAG | |
| β-actin | 150 | |
| Forward | TATCGGCAATGAGCGGTTCC3 | |
| Reverse | AGCACTGTGTTGGCATAGAGG3 |
3.11. Statistical Data Analysis
The normality of the data was assessed using the Shapiro-Wilk test. Since the data followed a normal distribution, one-way ANOVA was used for analysis, and Tukey's test was applied for pairwise comparisons between groups in SPSS 16.0 (SPSS Inc.). A P-value of < 0.05 was considered statistically significant. All data are presented as mean ± standard error of the mean (SEM). Diagrams were generated using GraphPad Prism 6.
4. Results
4.1. Pretreatment of Thyme Essential Oil on the Histopathology of Renal Tissue Following Renal Ischemia-Reperfusion
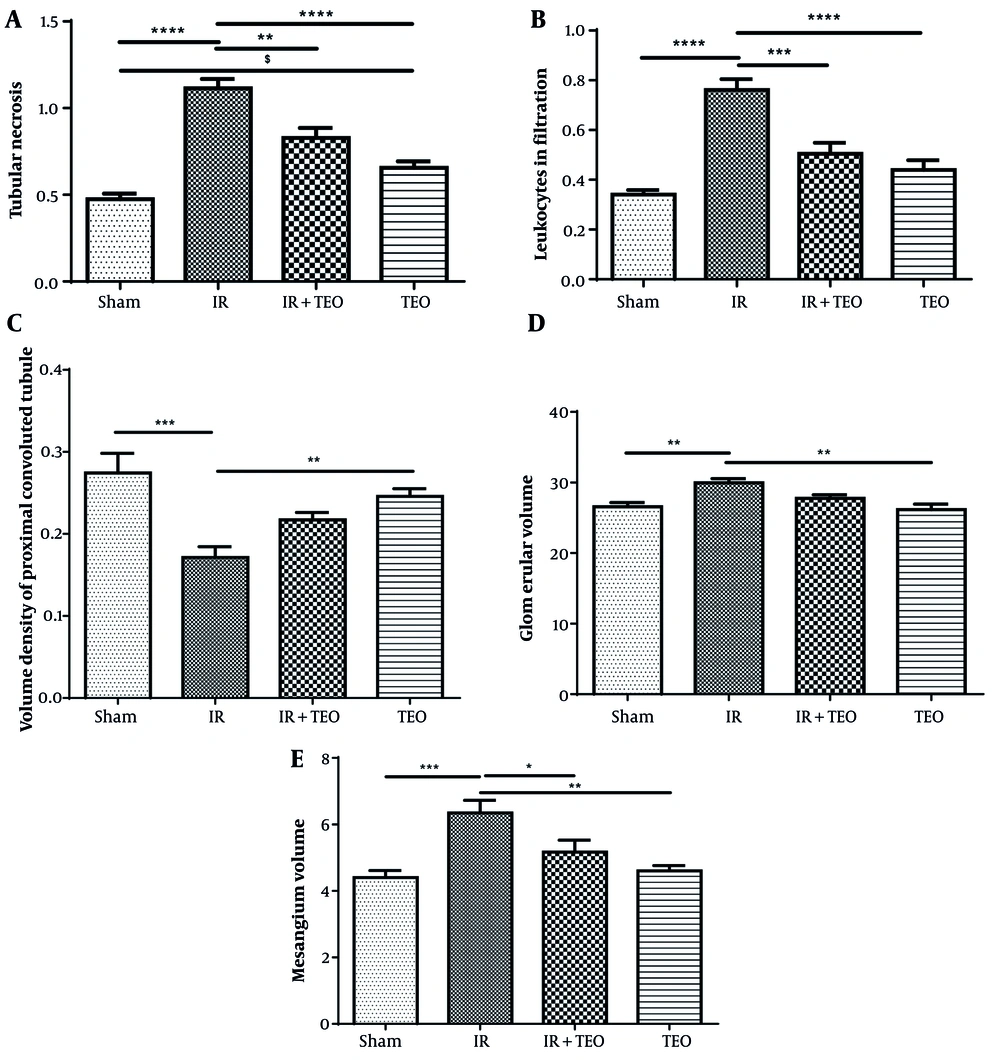
Tubular necrosis in the IR group significantly increased compared to the sham group (P < 0.0001). However, tubular necrosis in the IR+TEO group significantly decreased compared to the IR group (P < 0.01). In contrast, the TEO group showed a significant increase in tubular necrosis compared to the sham group (P < 0.05) (Figure 3A).
Effect of thyme essential oil (TEO) on histopathology of renal tissue following renal ischemia-reperfusion (IR). A, shows tubular necrosis; B, shows leukocyte infiltration; C, shows the volume density of the proximal convoluted tubule; D, shows the glomerular volume; and E, shows mesangium volume in different groups. Data were presented as mean + standard error mean (SEM). Data from this study were evaluated by analyzing using one-way ANOVA and, followed by Tukey test was used for comparisons between the two groups. A P-value is less than 0.05 was considered statistically significant. **** P < 0.0001 compared with IR, *** P < 0.001 compared with IR, ** P < 0.01 compared with IR, * P < 0.05 compared with IR. $ P < 0.05 compared with Sham. Pretreatment with TEO caused a significant decrease in tubular necrosis and leukocyte infiltration and a significant increase mesangium volume following renal ischemia-reperfusion (RIR).
Leukocyte infiltration in the IR group significantly increased compared to the sham group (P < 0.0001). However, the IR+TEO group exhibited a significant decrease in leukocyte infiltration compared to the IR group (P < 0.001) (Figure 3B).
The volume density of the proximal convoluted tubule in the IR group significantly declined compared to the sham group (P < 0.0001). However, no significant differences were observed between the IR+TEO group and the IR group or between the TEO group and the sham group (P > 0.05) (Figure 3C).
Glomerular volume in the IR group significantly increased compared to the sham group (P < 0.01), but no significant differences were found between the IR+TEO group and the IR group or between the TEO group and the sham group (P > 0.05) (Figure 3D).
The mesangium volume in the IR group significantly increased compared to the sham group (P < 0.001). However, the IR+TEO group demonstrated a significant reduction in mesangium volume compared to the IR group (P < 0.05) (Figure 3E).
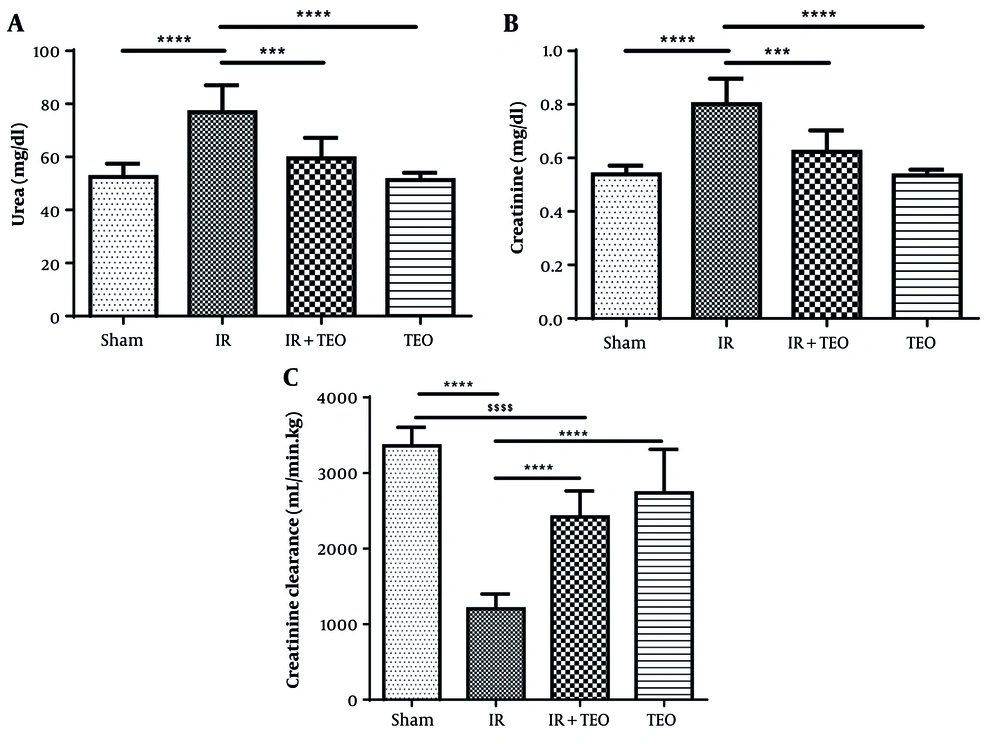
4.2. Pretreatment of Thyme Essential Oil on Renal Function Tests Following Renal Ischemia-Reperfusion
Serum urea levels in the IR group significantly increased compared to the sham group (P < 0.0001). However, serum urea levels in the IR+TEO group significantly decreased compared to the IR group (P < 0.001). No significant difference was observed between the sham and TEO groups in serum urea levels (P > 0.05) (Figure 4A).
Effect of thyme essential oil (TEO) on renal function test following renal ischemia-reperfusion (IR). A, shows serum urea; B, shows serum creatinine; and C, shows creatinine clearance in different groups. Data were presented as mean + standard error mean (SEM). Data from this study were evaluated by analyzing using one-way ANOVA and, followed by the Tukey test was used for comparisons between the two groups. A P-value is less than 0.05 was considered statistically significant. **** P < 0.0001 compared with IR, *** P < 0.001 compared with IR. $$$$ P < 0.0001 compared with Sham. Pretreatment with TEO caused a significant decrease in urea and creatinine and a significant increase creatinine clearance following renal ischemia-reperfusion (RIR).
Serum creatinine levels in the IR group significantly increased compared to the sham group (P < 0.0001). In contrast, serum creatinine levels in the IR+TEO group significantly decreased compared to the IR group (P < 0.001). No significant difference was found between the sham and TEO groups in serum creatinine levels (P > 0.05) (Figure 4B).
Creatinine clearance in the IR group significantly decreased compared to the sham group (P < 0.0001). However, creatinine clearance in the IR+TEO and TEO groups significantly increased compared to the IR group (P < 0.0001) (Figure 4C).
4.3. Pretreatment of Thyme Essential Oil on Biochemical Parameters Following Renal Ischemia-Reperfusion
The MDA levels in the IR group showed a significant increase compared to the sham group (P < 0.0001). However, MDA levels in the IR+TEO group significantly decreased compared to the IR group (P < 0.0001) (Table 2).
| Biochemical Parameters | Sham | IR | IR+TEO | TEO |
|---|---|---|---|---|
| MDA (μmol/mg proteins) | 169.13 ± 8.61 **** | 230.42 ± 5.99 ^^^^ | 158.34 ± 8.41 **** | 189.64 ± 8.00 |
| GSH (μmol/mg proteins) | 4.42 ± 0.22 ** | 3.38 ± 0.10 ^^ | 4.28 ± 0.17 ** | 4.43 ± 0.14 ** |
| GPX (U/ mg proteins) | 496.95 ± 0.10.35 **** | 404.98 ± 5.41 ^^^^ | 424.02 ± 8.88 ^^^^ | 430.42 ± 10.61 **** |
| CAT (U/ mg proteins) | 31.17 ± 0.90 **** | 12.51 ± 1.11 ^^^^ | 14.84 ± 0.96 ^^^^ | 25.62 ± 0.79 **** |
| NO (nmol/dl) | 4.18 ± 0.15 | 4.56 ± 0.11 | 4.30 ± 0.07 | 4.08 ± 0.08 * |
| MPO (U/ mg proteins) | 14.53 ± 0.43 **** | 20.19 ± 0.36 ^^^^ | 17.68 ± 0.81 ^ | 16.78 ± 1.01 * |
| PON1 (U/min) | 30.62 ± 0.53 **** | 14.31 ± 0.52 ^^^^ | 20.73 ± 0.48 **** | 24.05 ± 0.55 **** |
Abbreviations: IR, ischemia-reperfusion; TEO, thyme essential oil; MDA, malondialdehyde; GSH, glutathione; GPX, glutathione peroxidase; CAT, catalase; NO, nitric oxide; MPO, myeloperoxidase; PON1, paraoxonase 1.
a Data presented as mean ± SEM. Analyzed using one-way ANOVA followed by Tukey's test for group comparisons.
b P-value < 0.05 considered statistically significant.
c **** P < 0.0001 vs. IR, *** P < 0.001 vs. IR, ** P < 0.01 vs. IR, * P < 0.05 vs. IR. ^^^^ P < 0.0001 vs. Sham, ^^^ P < 0.001 vs. Sham, ^^ P < 0.01 vs. Sham, ^ P < 0.05 vs. Sham.
The GSH levels in the IR group significantly decreased compared to the sham group (P < 0.001). In contrast, GSH levels in the IR+TEO group significantly increased compared to the IR group (P < 0.01). No significant difference was observed between the sham and TEO groups in GSH levels (P > 0.05) (Table 2).
The GPX activity in the IR group showed a significant decrease compared to the sham group (P < 0.0001). However, no significant difference was observed between the IR and IR+TEO groups in GPX activity (P > 0.05) (Table 2).
CAT activity in the IR group significantly decreased compared to the sham group (P < 0.0001). No significant difference was found between the IR and IR+TEO groups in CAT activity (P > 0.05) (Table 2).
The PON1 levels in the IR group showed a significant decline compared to the sham group (P < 0.0001). However, PON1 levels in the IR+TEO group significantly increased compared to the IR group (P < 0.0001) (Table 2).
No significant difference was observed between the IR+TEO and IR groups or between the TEO and sham groups in NO levels (P > 0.05) (Table 2).
MPO levels in the IR group significantly increased compared to the sham group (P < 0.0001). However, no significant difference was found between the IR and IR+TEO groups in MPO levels (P > 0.05). Additionally, no significant difference was observed between the sham and TEO groups in MPO levels (P > 0.05) (Table 2).
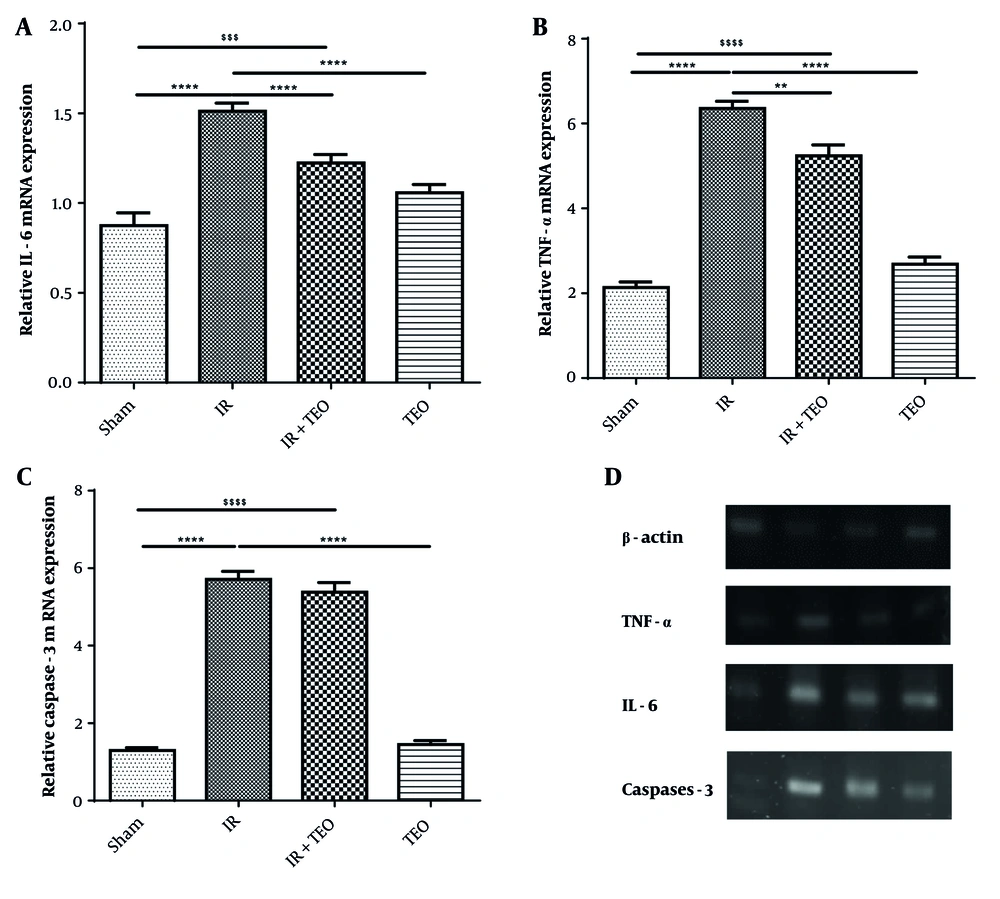
4.4. Pretreatment of Thyme Essential Oil on Gene Expression IL6, Tumor Necrosis Factor-α, and caspase-3 Following Renal Ischemia-Reperfusion
The mRNA expression of IL-6 in the IR group showed a significant increase compared to the sham group (P < 0.0001). However, IL-6 gene expression in the IR+TEO and TEO groups significantly decreased compared to the IR group (P < 0.0001) (Figure 5A).
Effect of thyme essential oil (TEO) on interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) and caspase-3 gene expression in the kidney following renal ischemia-reperfusion (IR). A, shows the mRNA expression of IL-6; B, shows the mRNA expression of TNF-α; and C, shows the mRNA expression of caspase-3 in different groups; D, mRNA expression of B-actin, TNF a, IL-6 and caspase-3. Data are presented as mean + standard error mean (SEM). Data from this study were evaluated by analyzed using one-way ANOVA and, followed by the Tukey test was used for comparisons between the two groups. The P-value is less than 0.05 was considered statistically significant. **** P < 0.0001 compared with IR, *** P < 0.001 compared with IR, ** P < 0.01 compared with IR. $$$$ P < 0.0001 compared with Sham, $$$ P < 0.001 compared with Sham. Pretreatment with TEO caused a significant decrease in renal TNF- α and IL-6 following renal ischemia-reperfusion (RIR).
The mRNA expression of TNF-α in the IR group demonstrated a significant rise compared to the sham group (P < 0.0001). A significant difference was observed between the IR and IR+TEO groups in TNF-α gene expression (P < 0.01). Additionally, the expression of TNF-α in the TEO group showed a significant decrease compared to the IR group (P < 0.0001) (Figure 5B).
The mRNA expression of caspase-3 in the IR group showed a significant increase compared to the sham group (P < 0.0001). However, no significant difference was observed between the IR and IR+TEO groups in caspase-3 gene expression (P > 0.05). In contrast, the expression of caspase-3 in the TEO group significantly decreased compared to the IR group (P < 0.0001). Moreover, no significant difference was found between the sham and TEO groups in caspase-3 gene expression (P > 0.05) (Figure 5C).
5. Discussion
The results generally indicated that the intraperitoneal injection of thyme oil as a pretreatment effectively reduced oxidative stress, increased antioxidant activity, decreased inflammation in renal tissue, and improved kidney function following RIR.
Various factors, including ROS, inflammation, and cellular apoptosis, contribute to renal damage caused by IR injury. Findings from previous studies suggest that ROS, reactive nitrogen species (RNS), and cytotoxic proteins such as MPO are major contributors to renal impairment during RIR (27, 29). A 24-hour reperfusion following 45 minutes of ischemia elevates pro-inflammatory cytokines, impairs renal function (e.g., increased serum BUN, creatinine, and uric acid), and induces structural changes in the kidney, including severe tubular necrosis, renal tissue congestion, swelling of tubular cells, tubular dilatation, leukocyte infiltration, and cellular vacuolization (30-32).
Additionally, DNA fragmentation, elevated cytochrome C levels, and increased caspase-3 activity indicate heightened apoptosis in kidney tissue after 45 minutes of ischemia and 24 hours of reperfusion (33, 34). Studies have also demonstrated that a 6-hour reperfusion following a 45-minute ischemia increases inflammation, oxidative stress, and apoptosis. Reperfusion generates ROS, which induces cell damage and kidney necrosis through multiple mechanisms, including membrane lipid peroxidation and DNA damage (35, 36).
In the present study, RIR-induced renal inflammation was associated with increased expression of inflammatory cytokines and apoptotic markers, highlighting the detrimental effects of ischemia-reperfusion injury on renal tissue.
Furthermore, RIR induced histopathological changes, including increased tubular necrosis, leukocyte infiltration, glomerular volume, and mesangium volume, along with a decrease in the volume density of the proximal convoluted tubule. The reduction in proximal convoluted tubule volume density and the increase in glomerular and mesangium volumes following RIR are attributed to tissue damage (10, 37).
Consistent with previous studies, our findings demonstrated that RIR led to a decline in the activity of antioxidant enzymes such as GPX and CAT, as well as a reduction in GSH and PON1 levels, while oxidative stress indices such as MDA, NO, and MPO were elevated (27, 38). Antioxidants and flavonoids have been shown to scavenge free radicals (39, 40). Natural antioxidants such as gallic acid, rosmarinic acid, coenzyme Q10, and glutathione play a crucial role in mitigating kidney damage by reducing oxidative stress, preventing renal tissue injury, and inhibiting inflammation via suppression of pro-inflammatory gene expression, including TNF-α and IL-1β (27, 37, 38, 41). Similarly, zinc oxide and ferulic acid have been reported to reduce oxidative stress, suppress inflammatory cytokine expression (TNF-α), and downregulate apoptotic genes such as caspase-3 and Bax, thereby minimizing tissue injury caused by ischemia-reperfusion (42).
Increased free radicals elevate lipid peroxidation, exacerbating tissue damage. Thyme oil and thyme powder have been reported to prevent liver damage by lowering lipid peroxidation levels (43). Additionally, thyme extract, rich in antioxidants, flavonoids, and polyphenols, has been shown to mitigate anxiety-like behaviors in animal models (44). Thyme oil protects against H₂O₂-induced cardiotoxicity by reducing lipid peroxidation and enhancing antioxidant activity (45). In a study evaluating the effects of thyme oil and rosemary on osteoporosis, thyme oil exhibited greater bone-forming and anti-inflammatory properties than rosemary (46). However, at a dose of 500 mg/kg orally for 28 days, thyme oil exhibited moderate toxicity, leading to severe lung tissue alterations (47).
Gholijani and Amirghofran reported that thymol and carvacrol, two major constituents of thyme oil, attenuate local inflammation (e.g., ankle inflammation) and reduce circulating inflammatory cytokines, including IL-17, IL-1β, and TNF-α (48). In our study, the intraperitoneal injection of TEO reduced oxidative stress, as evidenced by decreased MDA and MPO levels in renal tissue following RIR. Furthermore, increased GPX, GSH, CAT, and PON1 activity in the IR+TEO group confirmed the antioxidant potential of TEO.
Thyme essential oil, similar to rosmarinic acid, L-glutamine, olive leaf extract, and camphor, has been reported to alleviate pathological changes in renal tissue(10, 28, 37, 49). In the present study, renal tissue improvement was observed through reduced tubular necrosis, leukocyte infiltration, glomerular volume, and mesangium volume, along with increased proximal convoluted tubule volume density. Functional improvements were evidenced by decreased serum creatinine and urea levels, as well as enhanced creatinine clearance in the IR+TEO group.
Thyme extract is known to enhance immune function, modulate inflammation, and boost antioxidant defenses against oxidative stress, thereby contributing to overall health (50, 51). A study on Toxocara canis-infected testicular tissue demonstrated a relative decrease in caspase-3 expression following thyme administration (52). Similarly, thyme leaf extract mitigates methotrexate-induced toxicity by downregulating caspase-3 expression in testicular tissue (53).
The anti-inflammatory effect of TEO was evident in our study through reduced gene expression of pro-inflammatory cytokines IL-6 and TNF-α, leading to diminished inflammation in kidney tissue. However, TEO did not significantly reduce caspase-3 expression, which may be attributed to the presence of carvacrol in thyme oil. Carvacrol induces apoptosis by upregulating caspase-3 under hypoxic conditions, as observed in hypoxic pulmonary artery hypertension, where it limits vascular regeneration (54).
Previous reports suggest that oral administration of thyme at doses ranging from 0.5 to 3 g/kg is toxic, leading to liver damage, mucosal and skin irritation, respiratory distress, reduced motor activity, and increased mortality in animals. These toxic effects are primarily linked to the phenolic components of thyme, including carvacrol and thymol (55). In our study, intraperitoneal injection of thyme oil was used to circumvent mucosal damage. Additionally, a low-dose regimen was employed, ensuring no mortality among the animals.
5.1. Conclusions
Thyme essential oil, administered at a dose of 0.5 mL/kg via intraperitoneal injection, enhanced the kidney's antioxidant defense system, effectively reducing oxidative stress markers such as MPO and MDA. Additionally, TEO mitigated inflammation in the RIR model by downregulating the expression of inflammatory cytokine genes, including IL-6 and TNF-α. Therefore, in our study, TEO demonstrated protective effects against kidney damage in the RIR model due to its potent anti-inflammatory and antioxidant properties.