1. Background
Alterations in genes that govern cell death, proliferation, and differentiation can lead to cancer (1). Colorectal cancer (CRC) is the second and third most common cancer in women and men, respectively, and is one of the leading causes of cancer-related morbidity and mortality worldwide (2). The CRC is a slow-growing cancer that begins as a tissue growth in the lining of the colon or rectum (polyp). Abnormal and cancerous growth of polyps can lead to a tumor in the rectum or colon, with the potential to spread through blood vessels or lymphatic vessels, increasing the risk of metastasis (3). Several treatments for CRC are available depending on the size of the tumor, its location, and the patient's health, including surgery, cryosurgery, chemotherapy, radiation therapy, or targeted therapy (3, 4). Targeted treatment involves the use of drugs to attack cancer cells, inhibiting their proliferation, differentiation, and migration (3, 4).
Nanomedicine is the science of using nanotechnology to treat or diagnose diseases. Nanoparticles, which are 1 to 100 nanometers in size and smaller than biological organelles, can be used for therapeutic purposes and imaging due to their unique physical and chemical properties. Therapeutic and diagnostic nanoparticles offer better penetration and lower risk than conventional cancer treatments (5, 6). Selenium is a vital element that exhibits antioxidant properties via selenoproteins like glutathione peroxidase (GPx) and thioredoxin reductase when consumed in appropriate dietary amounts. However, selenium nanoparticles (SeNPs) exert various effects on cells by generating free radicals and oxidative stress, which can lead to cell death. Selenium presents an interesting contrast between antioxidant and pro-oxidant activities, depending on the dose and the cells exposed. Selenium compounds may induce oxidative stress and apoptosis (7).
Nanoparticles have a greater surface-to-volume ratio, resulting in enhanced chemical reactions and the formation of reactive oxygen species (ROS). Nanoparticles may induce toxicity through free radicals that cause oxidative stress, inflammation, and damage to proteins, cell membranes, and DNA. An increase in free radicals leads to the body's antioxidant defense response, which includes enzymes such as superoxide dismutase (SOD), GPx, and catalase (CAT) (8). Members of the Bcl2 family, such as Bax (pro-apoptotic) and Bcl2 (anti-apoptotic) genes, play a key role in regulating apoptosis. Additionally, the p53 gene, a pro-apoptotic protein, acts as a tumor suppressor, playing a vital role in tumor suppression by inducing apoptosis and halting the cell cycle (9). To determine the effect of SeNPs, the activity levels of SOD, GPx, and CAT enzymes, ROS levels, and the regulatory genes related to cancer, Bax, Bcl-2, and P53, were examined (10).
2. Methods
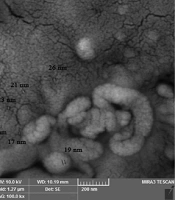
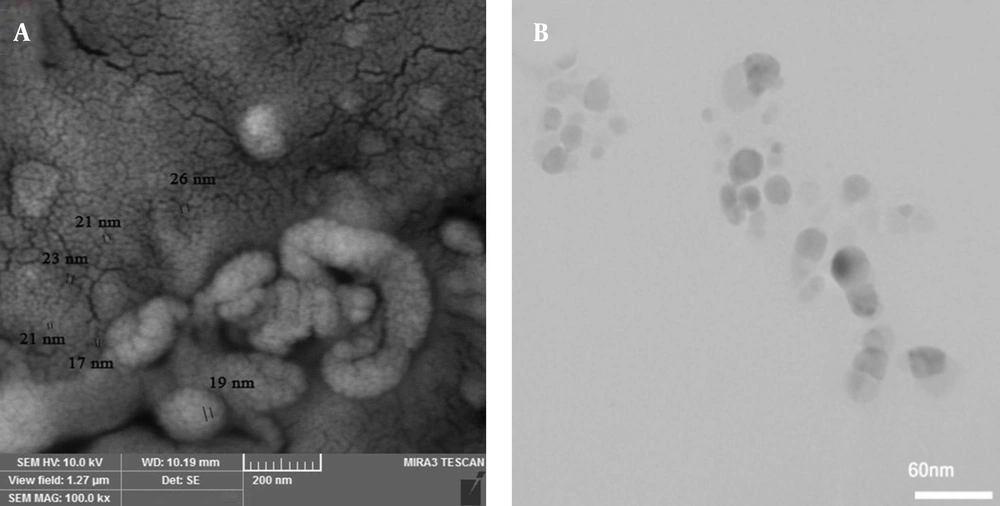
Selenium nanoparticles with 99.95% purity, 30 nm in size, and a concentration of 1000 ppm were purchased from NANOSANY Company (Mashhad, Iran). Figure 1 shows the electron microscope micrograph of SeNPs.
2.1. Treatment of the SW480 Cell Lines
The SW480 human colon cancer cell line was obtained from The Pasteur Institute (Tehran, Iran). The cells were cultured under standard conditions in RPMI-1640 medium containing 10% fetal bovine serum and 1% penicillin-streptomycin. The cells were maintained at 37°C in an incubator with 5% CO2. Initially, SW480 cells were seeded at a density of 11 × 103 cells/well in a sterile 96-well plate. After 24 hours of incubation, the cells were exposed to a range of selenium nanoparticle (SeNP) concentrations (0, 5, 10, 20, 50, 75, 100, 125, 200 µg/mL) (11) Control cells were treated with RPMI-1640 medium. After 24 hours of treatment, the cytotoxicity of the SeNPs on the cells was assessed using the MTT assay.
2.2. Cytotoxicity Assessment
MTT refers to the compound 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. The MTT assay was conducted to study the effect of SeNPs on cells in three main stages: seeding, treatment, and MTT assay. After the cells were treated with nanoparticles for 24 hours, 20 µL of MTT solution was added to each well. The plate was incubated in the dark for 4 hours, after which the supernatant of each well was removed. Formazan crystals were then dissolved with 100 µL of dimethyl sulfoxide (DMSO). Following a 30-minute incubation, optical density (OD) was measured using an enzyme-linked immunosorbent assay (ELISA) plate reader (RT-2100C Microplate Reader, China) at 570 nm. Finally, cell viability was calculated using the formula: (OD of SeNP-treated cells/OD of control cells) × 100. The IC50 (50% inhibitory concentration) was determined using GraphPad Prism 9 software.
2.3. Measurement of Reactive Oxygen Species
The effect of SeNPs on intracellular ROS levels was assessed using a fluorometric assay, following the manufacturer's instructions (Kiazist, Iran). Briefly, cells were seeded at a density of 22 × 103 cells per well. After 24 hours of incubation, the plate was exposed to selected concentrations of SeNPs (0, 75, 97, and 125 μg/mL). Following a 24-hour incubation, the supernatant was withdrawn from the wells. Then, 100 μL of 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) solution was added to each well, and the cells were incubated at 37°C for 45 minutes. Immediately after incubation, fluorescence was measured at Ex/Em (528/485 nm) using a Multi-Mode Reader (Bio-Tek Instruments, Winooski, USA). The increase in ROS compared to untreated cells was reported as fold change or multiple changes.
2.4. Evaluation of Antioxidant Enzyme Activities
Kits for the determination of SOD (KSOD-96), GPx (KGPx-96), and CAT (KCAT-96) were purchased from Kiazist Company, Iran. Enzyme activities were evaluated according to the manufacturer's protocol. The SOD kit is based on the transformation of purple to blue resazurin into pink resorufin in the presence of O2. The GPx enzyme, with the aid of glutathione as an electron donor, regenerates the active form of selenocysteine at its active site. The peroxidation activity of CAT with methanol results in the production of formaldehyde, which reacts with Purpald to produce a purple dye. Absorption was measured at 540 nm using a UV/Vis spectrophotometer (BEL, LGS 53, Italy).
2.5. Study of Genes Expression by qRT‑PCR
Total RNA from control and treated SW480 cells was isolated using RNX-plus™, following the supplier's instructions. The quality of the isolated RNA was evaluated using a NanoDrop One UV – Vis Spectrophotometer (Thermo Fisher Scientific, USA) and 1% agarose gel electrophoresis. cDNA synthesis was performed using a kit from Pars Tous Company (Iran). Subsequently, quantitative real-time PCR (qRT-PCR) was conducted on a LightCycler® 96 System (Roche Diagnostics Corporation, Germany) using the cDNA template, forward and reverse primers (Table 1), SYBR Green qPCR master mix (Ampliqon, Denmark), and DNase/RNase-free water. Primer sequences for Bax, Bcl2, and p53 were designed using Primer3 software. The β-actin gene was used as the reference gene. Gene expression levels were presented as fold change relative to the control.
| Genes | Primers |
|---|---|
| ACTB | |
| Forward | 5´-TAA CGC AAC TAA GTC ATA-3´ |
| Reverse | 5´-AAGATC AAG ATC ATT GCT-3´ |
| P53 | |
| Forward | 5´-TGTGACTTGCACGTACTCCC-3´ |
| Reverse | 5´-ACCATCGCTATCTGAGCAGC-3´ |
| Bax | |
| Forward | 5´-GGTTGTCGC CCT TTT CTA CTT-3´ |
| Reverse | 5´-GGA GGAAGT CCA ATG TCC AG-3´ |
| Bcl2 | |
| Forward | 5´-GAGCGTCAACCGGGAGATGT-3´ |
| Reverse | 5´-TACAGTTCCACAAAGGCATCCCAG-3´ |
2.6. Data Analysis
Analyses were performed using GraphPad Prism version 9, and data are presented as mean ± standard deviation (SD) from three independent experiments (n = 3). A P-value of less than 0.05 was considered statistically significant.
3. Results
3.1. The SW480 Cell Lines Viability
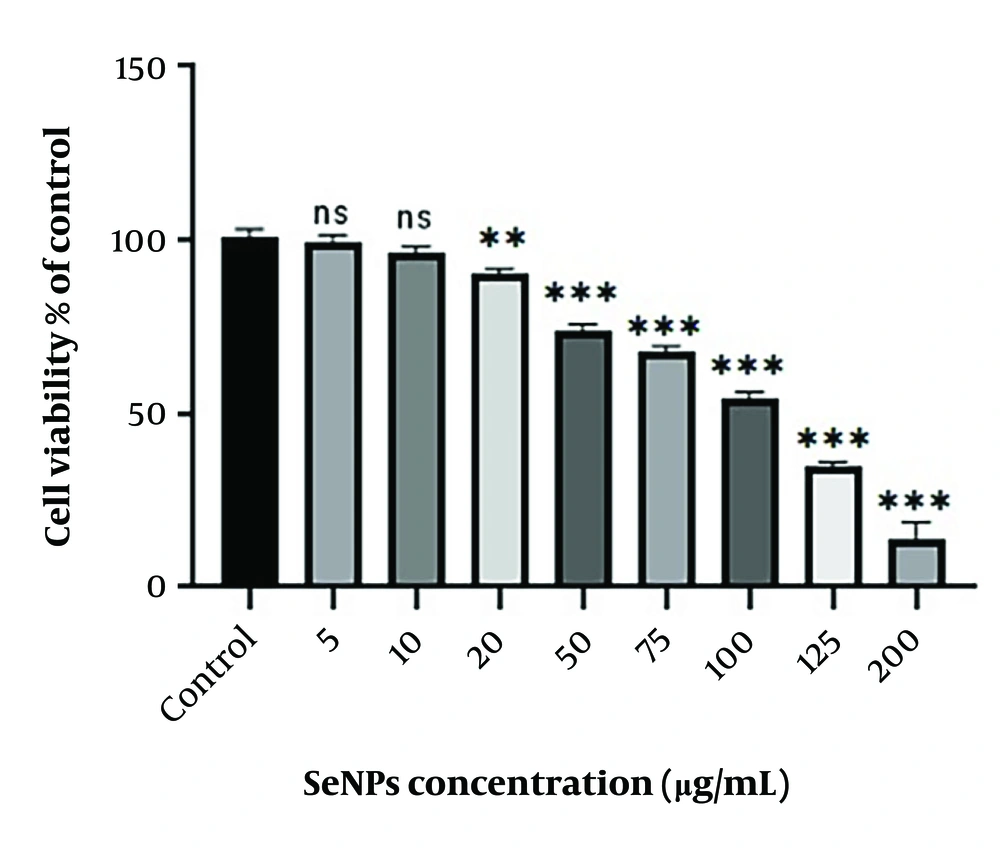
The treatment of SW480 cells with SeNPs at concentrations of 0, 5, 10, 20, 50, 75, 100, 125, and 200 µg/mL for 24 hours was assessed using the MTT assay. The findings, as shown in Figure 2, demonstrated no discernible difference in cell viability between the groups treated with doses of 5 and 10 µg/mL and the control group. However, doses ranging from 20 to 200 µg/mL significantly reduced cell viability in a dose-dependent manner, with the IC50 calculated as 97 µg/mL. Concentrations of 75, 97, and 125 µg/mL were selected for further experiments.
3.2. Effects of Selenium Nanoparticles on Reactive Oxygen Species Production
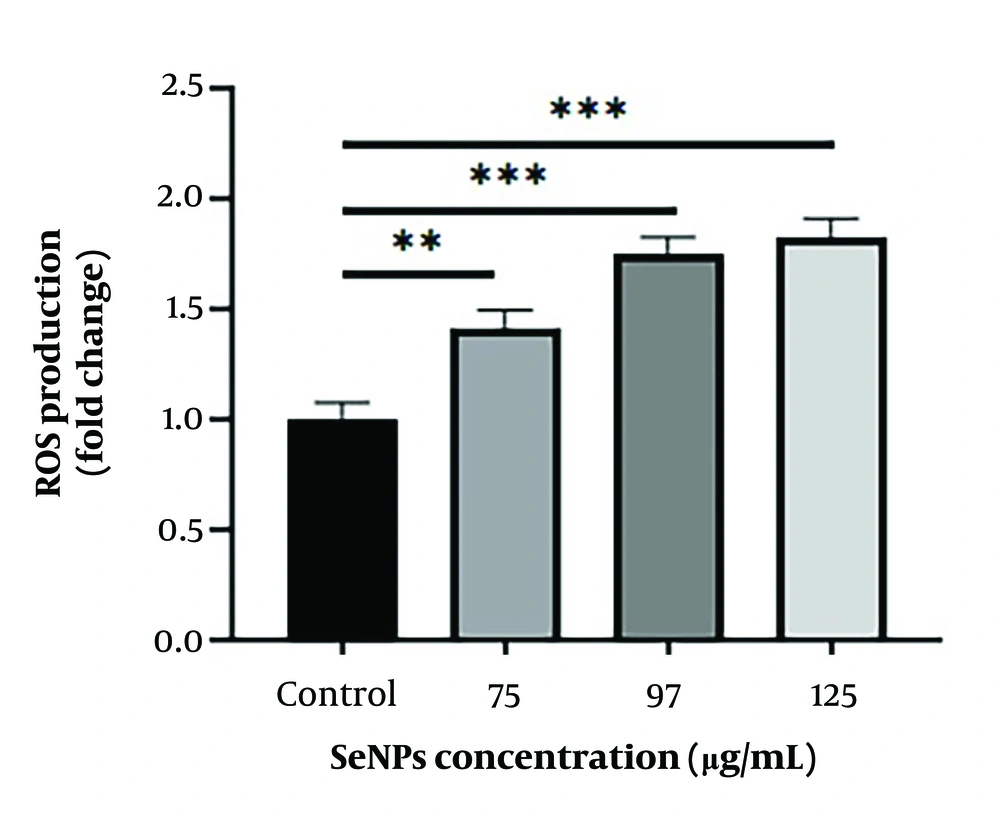
Intracellular ROS levels were measured using a fluorimetric method to further investigate the ROS-dependent apoptotic pathway. As shown in Figure 3, treatment of SW480 cells with SeNPs for 24 hours resulted in increased ROS production. The levels of ROS in cells treated with concentrations of 75 µg/mL (P < 0.01), 97 µg/mL, and 125 µg/mL (P < 0.001) were significantly higher compared to untreated cells.
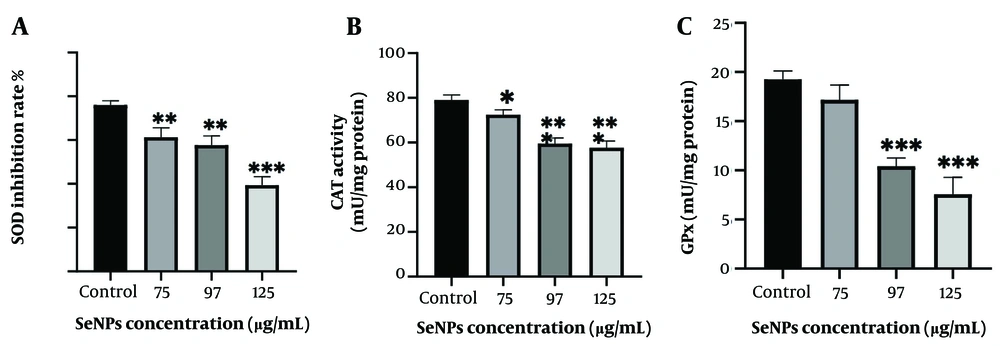
3.3. Effects of Selenium Nanoparticles on the Activity of Antioxidant Enzymes
As shown in Figure 4, the activity of the SOD enzyme (Panel A) was significantly decreased in groups treated with SeNPs at concentrations of 75 µg/mL and 97 µg/mL (P < 0.01) compared to untreated cells. Moreover, a substantial reduction in activity was observed at a concentration of 125 µg/mL (P < 0.001). In Panel B, the graph shows that the level of CAT enzyme activity was reduced in cells treated with concentrations of 75 µg/mL, 97 µg/mL, and 125 µg/mL of SeNPs compared to the control group (P < 0.005, P < 0.001, and P < 0.001, respectively). Additionally, the activity of the GPx enzyme in cells treated with 97 µg/mL and 125 µg/mL SeNPs changed significantly compared to untreated cells (P < 0.001), while no significant change was observed in GPx activity in cells treated with a 75 µg/mL concentration (Panel C).
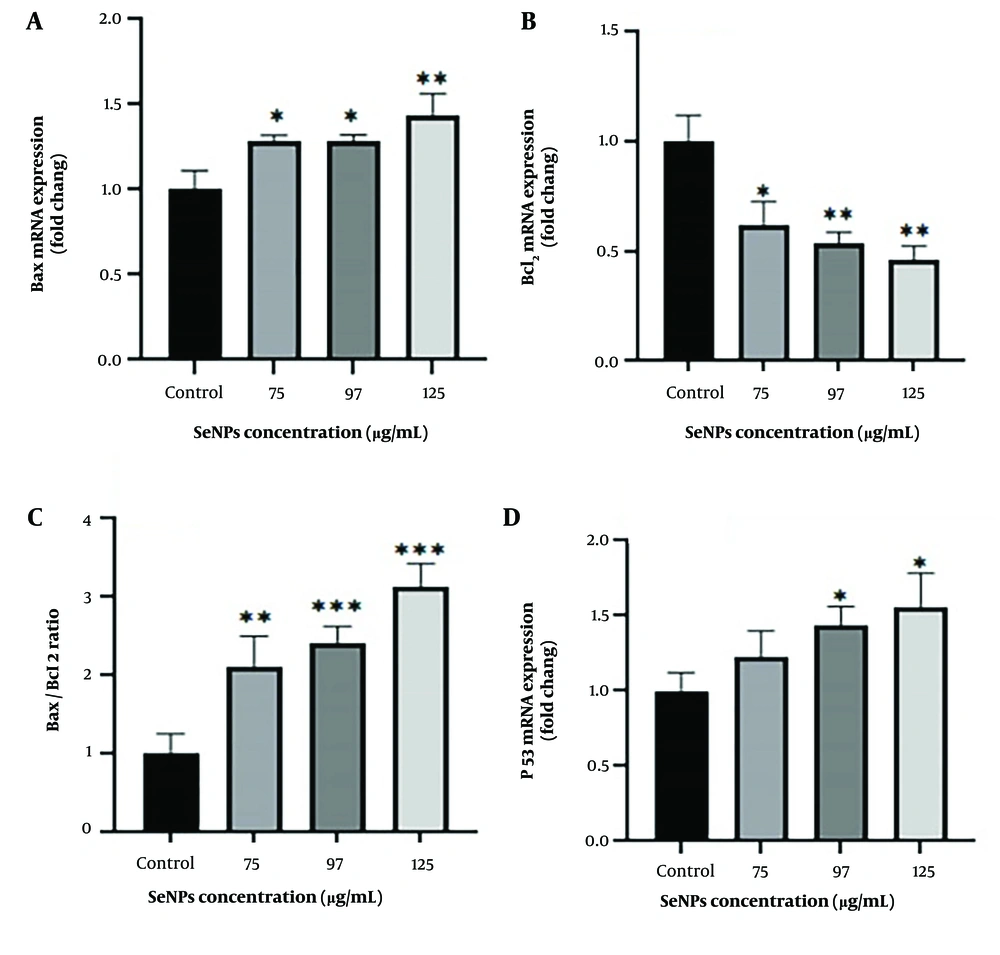
3.4. Effects of Selenium Nanoparticles on the Expression of Apoptotic Genes
As shown in Figure 5A, a significant increase in Bax expression was observed at concentrations of 75 µg/mL and 97 µg/mL (P < 0.05) and 125 µg/mL (P < 0.01). In contrast, a notable decrease in the expression of Bcl2 was observed at concentrations of 75 µg/mL, 97 µg/mL, and 125 µg/mL compared to untreated cells (Figure 5B). Furthermore, the expression of the p53 gene was evaluated, and Figure 5C shows a significant increase in cells treated with concentrations of 97 µg/mL and 125 µg/mL (P < 0.05).
4. Discussion
The CRC, a common malignancy worldwide, has been one of the leading causes of mortality in recent years (4). The use of nanotechnology holds promise for improving the diagnosis and treatment of CRC (12). The absorption or expulsion of nanoparticles by cells largely depends on their size. Additionally, the internalization and therapeutic qualities of nanoparticles are enhanced by their shape. Studies indicate that nanoparticles interact with the cell membrane to gain entry (13). Selenium is an important element in biochemistry and physics, found in nature with various structures. Most selenium-containing proteins in the body have enzymatic functions. Selenium plays a key role in strengthening the immune system, metabolism, thyroid function, and cancer prevention, among other processes. However, high levels of selenium can have toxic effects (14, 15).
Selenium can be effective in higher doses for inhibiting cancer, though the mechanism varies depending on the cell type and selenium species. The use of nanoparticles in treating many diseases, especially cancer, is emerging as a more optimal alternative. The SeNPs show promising potential as therapeutic agents and drug carriers. Nanoparticles may enter cells via passive or active transport and exit through transcytosis or exocytosis. The transfer, absorption, or elimination of these nanoparticles from the human body is influenced by factors such as surface properties, shape, and size (16-18).
The effect of SeNPs on the colorectal adenocarcinoma cell line SW480 was investigated. The results showed that the dose-dependent cytotoxicity of SeNPs in SW480 cells decreased cell viability, with an IC50 of 97 µg/mL. Additionally, ROS production increased in SW480 cells after exposure to SeNPs, affecting the expression levels of studied genes. Previous studies have shown that SeNPs can be toxic and may induce apoptosis by increasing cellular ROS (19-21). Menon and Shanmugam reported on the viability of A549 cell lines treated with different concentrations of SeNPs for 24 and 48 hours, concluding that cell viability was reduced in a time- and dose-dependent manner. The possible mechanism of SeNPs' cytotoxicity is related to their passage through ion channels into the cell membrane and interaction with intracellular proteins or nitrogenous bases in DNA (11).
Furthermore, the relative anticancer effects of SeNPs were studied in breast cancer cells (MCF-7) and lymphoma cancer cells (Raji Burkitt cells). The results showed that SeNPs possess significant anti-proliferative properties against MCF-7 and Raji Burkitt cell lines. The SeNPs demonstrated a greater effect against MCF-7 cells compared to Raji Burkitt lymphoma cell lines (22). Many studies have shown that therapeutic approaches aimed at lowering ROS or increasing its products are likely to be promising and successful treatments for malignancy (23). Given the importance of measuring ROS in cancer cells and developing antitumor drugs based on it, the current report assessed the potential of SeNPs to increase ROS levels using a fluorimetric method. In line with our results, Tang et al. evaluated the amount of ROS produced by HepG2 cancer cells during treatment with SeNPs, finding that cellular ROS levels were greatly increased compared to the control group. Reactive oxygen species generation may become excessive in HepG2 cells due to the redox metabolism of SeNPs. The SeNPs caused mitochondrial dysfunction and raised ROS levels compared to the control (24).
Nanoparticles containing selenium accumulate in tumor tissues by increasing the enhanced permeability and retention (EPR) effect, thereby killing cancer cells and inhibiting tumor growth (25). Although the cellular mechanism of ROS production due to nanoparticles is still unknown, its production mechanism varies among cells. Antioxidant enzymes play an important role in maintaining normal metabolism and functioning of organisms; therefore, antioxidant indices, including the activity of CAT, GPx, and SOD enzymes, were evaluated. Based on the present results, another study showed that the activities of SOD and GPx enzymes were significantly reduced after treatment with SeNPs in IPEC-J2 cells (26).
To evaluate the effect of SeNPs on apoptosis, qRT-PCR was used. In addition to the inhibitory effects of SeNPs on the survival of SW480 cells, the results of the present study indicate the induction of apoptosis in the presence of these nanoparticles. The current study's results, which examine alterations in the expression of the p53, Bax, and Bcl2 genes, validate that SeNPs induce apoptosis. Evidence suggests that nanoparticles can re-regulate the expression of genes related to apoptosis, reduce the integrity of the mitochondrial outer membrane, and activate caspases (27, 28). Based on our results, some studies showed that SeNPs change the expression of pro-apoptotic genes p53, Bax, and Bcl2 (29-31). Altogether, it seems possible to achieve promising results for cancer treatment by further studying SeNPs.
4.1. Conclusions
The results obtained from this study demonstrated that SeNPs can reduce the survival of SW480 cancer cells in a concentration-dependent manner. It was observed that SeNPs at high doses have the ability to reduce antioxidant capacity and increase oxidative parameters in colon cancer cells. The induction of apoptosis in cells treated with SeNPs was confirmed by the elevated formation of ROS, a significant increase in the expression of Bax and p53, and a notable decrease in Bcl2 expression.