1. Background
Periventricular leukomalacia (PVL) is the most common type of ischemic brain injury of white matter observed in preterm infants, particularly those born before 36 weeks’ gestation (1). Cerebral palsy (CP) is a long-lasting, non-progressive condition marked by brain damage and alterations in neuropsychomotor development. The CP is the most common factor contributing to motor impairment in children worldwide, affecting 1.5 to 4 out of every 1,000 children. In low-resource areas, it affects up to 10 out of every 1,000 children (2). The CP is categorized based on the type and location of motor problems and the area of brain damage, including spastic (bilateral-quadriparetic, -diparetic, or unilateral-hemiparetic), dyskinetic, and ataxic subtypes (3). The causes of CP are varied, as are the kind and degree of associated motor and neurological impairment. Clinical characteristics such as kernicterus, dyskinetic CP, and persistent newborn hyperbilirubinemia have been consistently linked to etiology and pathology (4). A more common link is PVL and premature delivery. Cerebral palsy and cognitive impairments in preterm newborns are mostly due to PVL (5).
Brain injury in newborns can manifest in many ways, making early identification of neonatal encephalopathy risk difficult (6). Neonatal encephalopathy is projected to occur 3 times per 1,000 live births (95% CI 2.7 - 3.3), and hypoxic-ischemic encephalopathy 1.5 times per 1,000 live births. Hypoxic-ischemic encephalopathy affects 1 - 8 per 1,000 live births (7). Intrapartum hypoxic ischemia is found in 30% of established newborn encephalopathy cases and 60% of developing cases (8). The most prevalent childhood physical disability is CP, occurring at 2 - 2.5 per 1,000 live births in affluent nations (9).
In healthy individuals, glial fibrillary acidic protein (GFAP) is the primary component of intermediate filaments in the cytoplasm of glial cells, notably astrocytes (10). Changes in GFAP values precisely characterize brain functions. The GFAP is a structural protein not released from cells under physiological conditions, and healthy individuals have undetectable blood levels (11). The GFAP is released into plasma when astroglial cells undergo necrosis. In intracerebral hemorrhage (12) and severe brain injury (13), GFAP is found early. The GFAP levels are significantly higher than usual in patients with severe brain injury. High GFAP levels (> 0.08 µg/L) are linked to poor prognosis (14).
Calcium (Ca2+) is crucial to glutamatergic synaptic signal transduction (15). In glutamatergic synapses, Ca2+ spike patterns serve as the source of information, necessitating protein machinery to decode them into long-lasting biochemical and cellular changes that alter memory (16). Calmodulin, a calcium marker in eukaryotic cells, forms the Ca2+/calmodulin complex with calcium ions. This complex regulates cell proliferation and differentiation by binding to and inhibiting the autoinhibition domain of protein kinases such as myosin light chain kinase, Ca2+/calmodulin-dependent kinase, protein kinase C (PKC), and adenylate cyclase. Ca2+/calmodulin-dependent kinases are crucial to the G1 to S phase cell cycle transition, and many cell pathways depend on calmodulin (17).
Gelsolin, a calcium-dependent multifunctional actin-regulating protein, is found in humans. Gelsolin rapidly severs and removes actin filaments released into the bloodstream by dying cells (18). Gelsolin also binds proinflammatory and bioactive molecules such as lysophosphatidic acid, sphingosine 1-phosphate, fibronectin, and platelet-activating factor, which regulate wound healing, neurological growth, cancer spread, and angiogenesis. Cells express more gelsolin in response to oxidative stress, suggesting it may act as an antioxidant (19). Low gelsolin levels have been linked to bronchopulmonary dysplasia, hyperoxic lung damage, sepsis, and cerebral hemorrhage. Gelsolin is also associated with long-term problems that develop in premature infants (20).
Piracetam, first developed between the 1950s and 1964 by Romanian chemist and psychologist Corneliu E. Giurgea, has been used to treat epilepsy since the 1950s and is widely available and easy to obtain (21). Piracetam may protect neurons harmed due to inflammatory processes that damage cells and molecules during aging, but more research is needed (22). Given the high rate of preterm birth and its complications, including PVL, intervention should be initiated as soon as feasible in the womb.
2. Objectives
The present study aimed to investigate the impact of piracetam on the mRNA expression of GFAP, calmodulin 2, and gelsolin in the embryonic brains of pregnant rats (Figures 1 and 2). The objective was to assess the protein levels and mRNA expression of GFAP, calmodulin-2, and gelsolin in pregnant rat models of PVL, to determine whether piracetam can prevent the occurrence of CP in prematurely born rat offspring.
3. Methods
3.1. Protocol Animals
The research was conducted exclusively with a post-test control group. A pregnant rat (Rattus norvegicus) model of PVL was used, involving rats aged 10 weeks and weighing 150 - 200 g, housed with adequate humidity and lighting. Twenty female Wistar rats (R. norvegicus) weighing 240 - 250 g and ten males weighing 290 - 300 g were kept in a normal animal center maintained at 23 ± 2°C and 55% humidity. They had free access to food and water. Throughout the study, there was a daily cycle of 12 hours of light and 12 hours of dark. After one week of adapted food consumption, the researchers introduced the rats to tandem enclosures, pairing one male with every two females. Vaginal smear screenings began the next day, and the sperm plug was found on the first day of pregnancy.
An animal model of PVL was created by intracervical injection of lipopolysaccharide (LPS) at a dose of 1 mg/kg body weight (BW). The minimum sample size was calculated using the G*Power application. Sixty-three pregnant rats were randomly assigned into two groups: The control and treated groups. The control group was divided into normal pregnant rats (K0) without LPS injection, those injected with LPS (K1), and a group of pregnant rats injected with distilled water as a placebo (K2). The treatment groups were all injected with LPS and given piracetam orally from day 10 to day 19 at different doses: Fifty mg/kg BW (P50), 100 mg/kg BW (P100), 150 mg/kg BW (P150), and 200 mg/kg BW (P200). After the administration of piracetam on day 19, all rats were born to obtain premature rats.
3.2. Examination Protein Molecular
The intracervical injection of LPS at 1 mg/kg BW was administered on days 15, 17, and 19. On day 19, the pregnancies were interrupted to deliver premature rat fetuses, as shown in Figure 1. The brain tissues of the fetuses (Figure 2) were collected and examined using ELISA and real-time PCR to assess the molecular proteins GFAP, calmodulin-2, and gelsolin.
3.3. Statistical Analysis
The post-hoc parametric test used was the one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) multiple comparison test. Non-parametric tests such as the Mann-Whitney and Kruskal-Wallis tests were employed. A difference was considered statistically significant if the P-value was less than 0.05.
4. Results
4.1. Examination of Protein Levels and mRNA Expression of GFAP
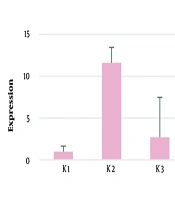
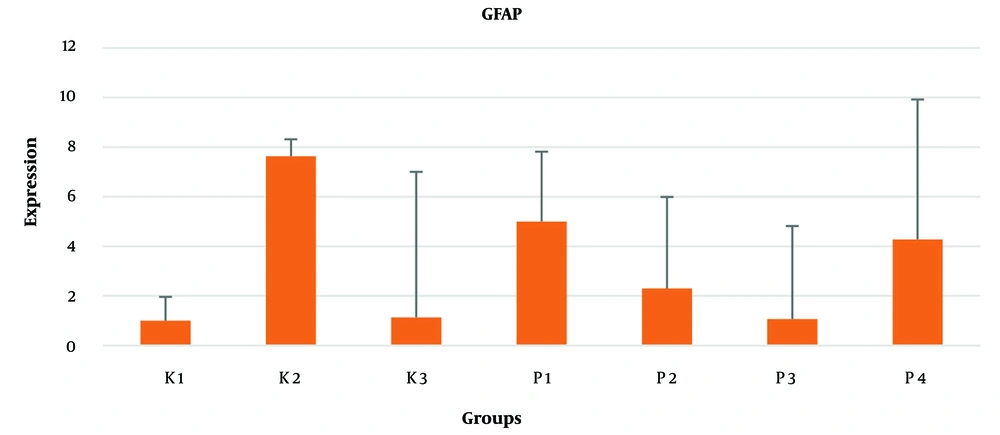
This study found that GFAP mRNA and protein levels increased in the PVL model rat group but showed a decrease after the administration of piracetam. Statistically, there was no significant difference in protein and mRNA levels of GFAP in fetal brain tissue between the control and treatment groups (P > 0.05) (Table 1 and Figure 1).
| Groups | Median (Min-Max) | P-Value |
|---|---|---|
| K1 | 5.62 (0 - 6) | - |
| K2 | 6.35 (5 - 9) | - |
| K3 | 5.59 (3 - 6) | 0.886 a |
| P1 | 4.09 (4 - 5) | - |
| P2 | 4 (3 - 5) | - |
| P3 | 3.65 (3 - 4) | - |
| P4 | 3.43 (3 - 4) | - |
Glial Fibrillary Acidic Protein Examination (pg/mL) in Fetal Brain Tissue of Pregnant Rats with Periventricular Leukomalacia Model
4.2. Examination of Protein Levels and mRNA Expression of Calmodulin-2
There was an increase in calmodulin-2 levels (pg/mL) in pregnant rats injected with LPS, and there was a significant difference between the control and the treatment group (P < 0.05). The treatment group showed a decrease in calmodulin-2 levels (pg/mL) across all doses of the piracetam-treated groups. There was a significant difference between the control group (K1) and all treatment groups (P50, P100, P150, P200) (P < 0.05) (Table 2).
| Groups | Median (Min-Max) | P-Value | K2 | K3 | P1 | P2 | P3 | P4 a |
|---|---|---|---|---|---|---|---|---|
| K1 | 336 (23.5 - 921.50) | 0.546 | 0.063 | 0.666 | 0.190 | 0.489 | 0.019 | |
| K2 | 288.1 (561 - 381.2) | 0.006 a | 0.489 | 0.113 | 0.546 | 0.011 | ||
| K3 | 544.4 (354.8 - 742.3) | 0.000 b | 0.063 | 0.006 a | 0.003 a | 0.000 | ||
| P1 | 488.1 (23.50 - 767.3) | 0.258 | 0.436 | 0.001 | ||||
| P2 | 161 (17.3 - 713.1) | 0.387 | 0.005 | |||||
| P3 | 256.9 (73.5 - 659.) | 0.0024 | ||||||
| P4 | -1.5 (-36.9 - 409) |
Calmodulin-2 (pg/mL) in Fetal Brain Tissue in Pregnant Rats of Periventricular Leukomalacia Model of Control and Treatment Groups
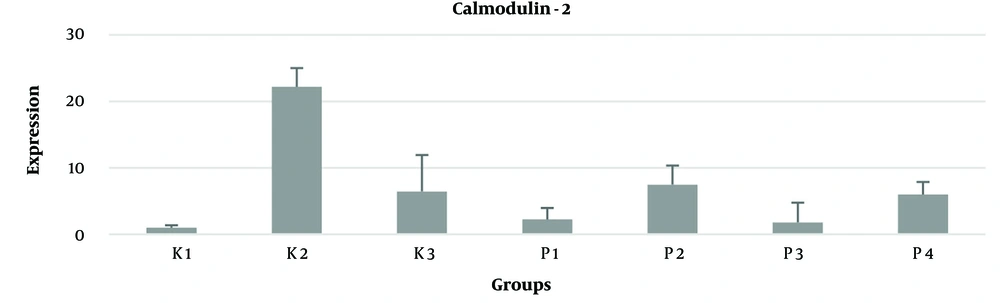
There was an increase in calmodulin-2 mRNA expression in the PVL model rat group, but this decreased after administering piracetam. A decrease in calmodulin-2 mRNA expression (pg/mL) was observed with the administration of piracetam at all doses (50, 100, 150, 200 mg) in the K2 control group. The treatment groups with piracetam at 50 mg and 100 mg dosages showed calmodulin mRNA expression levels close to the control group K0, as shown in Figure 2.
4.3. Examination of Protein Levels and mRNA Expression of Gelsolin
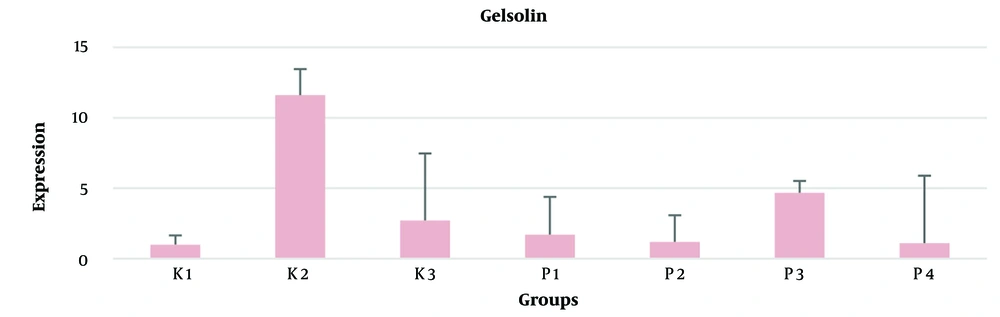
There was an increase in gelsolin levels (pg/mL) in all the pregnant rats injected with LPS, and a decrease in gelsolin levels in all the treatment groups at all doses of piracetam. The statistical tests showed no significant differences between the control and treatment groups (P > 0.05) as indicated in Table 3. Gelsolin mRNA expression showed an increase in the PVL rat model group but decreased expression after the administration of piracetam. However, this decrease in mRNA expression was not statistically significant (P > 0.05) as shown in Figure 3.
| Groups | Median (Min-Max) | P-Value |
|---|---|---|
| K1 | 202.70 (24 - 307) | - |
| K2 | 454.75 (288 - 561) | - |
| K3 | 37.05 (62 - 544) | 0.686 a |
| P1 | 57.90 (24 - 463) | - |
| P2 | 94.35 (49 - 161) | - |
| P3 | 160 (74 - 244) | - |
| P4 | 74.60 (37 - 409) | - |
Gelsolin (pg/mL) in Fetal Brain Tissue from Pregnant Rats Periventricular Leukomalacia Model
5. Discussion
The research objective was to examine the effect of the drug piracetam in preventing CP in fetuses using a pregnant rat model of PVL. The use of LPS induces PVL in pregnant rats. This method has been employed by previous researchers through intrauterine administration of LPS to pregnant rats (23). The GFAP levels are a biomarker that correlate with the severity of intracranial damage and can also be used to detect injury to the central nervous system (24). In this study, there was an increase in GFAP levels in the group injected with LPS (K1) compared with groups K0 and K2, and reduced GFAP levels in the treatment group at all doses of piracetam. In this context, piracetam may be used to reduce GFAP levels and aid in the prevention of CP in fetuses born from pregnant rats induced into PVL. However, this reduction was not statistically significant (P > 0.05). Clinically related examinations of GFAP levels, such as in patients with neonatal encephalopathy (25) or unipolar depression, reveal an increase in cerebrospinal GFAP levels, but a decrease in these levels in cases with healing (26). However, this study is not directly relevant because it examined GFAP levels in brain tissue.
In the present study, it was found that the levels of calmodulin-2 in fetal brain tissue differed significantly between the control and treatment groups (P < 0.05). Calmodulin-2 levels in the K0 group were higher than in the K1 control group, and the K2 control group had higher calmodulin-2 levels than the other two control groups. There was a significant difference between all control groups (K0, K1, K2) and the intervention group with the 200 mg dose (P200) (P < 0.05).
In addition to the significant difference in calmodulin-2 levels with the treatment group (P200), there were also significant differences in the treatment groups (P100, P150) compared to the K2 control group. In this study, the piracetam dose of 50 mg resulted in higher levels of calmodulin-2 compared to the groups receiving doses of 100, 150, and 200 mg. The calcium sensor calmodulin has many roles in neurons, including signaling through calmodulin-dependent kinases, regulation of ion channels, and others. Researchers have shown that calmodulin contributes to the control of gene expression that affects synapse formation and brain development, and the specification of synaptic properties that vary between the forebrain and hindbrain (27).
In this study, there were also higher levels of gelsolin in the LPS injection control group (K1) compared to the control groups (K0, K2). Gelsolin levels in all treatment groups (P50, P100, P150, and P200) were lower than those of the control groups (K0, K1). However, all treatment groups had higher levels compared to the placebo control group (K2). Other research shows that decreases in gelsolin levels strongly correspond with the amount of time needed to experience hypothermia and may predict the severity of hypoxic-ischemic encephalopathy, indicating the predictive value of plasmatics (28).
There are no studies explaining the relationship of gelsolin levels to CP, but research evaluating gelsolin levels in plasma showed reduced levels in neonatal hypoxic-ischemic encephalopathy (HIE), and reduced gelsolin levels can also predict the severity of HIE. Gelsolin has different protective roles in disease states, including protection against bioactive compounds and circulating proinflammatory agents such as fibronectin, LPS, lysophosphatidic acid, sphingosine 1-phosphate, and platelet activation factor (29). The levels of gelsolin in all treatment groups were lower than in the K1 control group. The very high levels of gelsolin protein in the control group (K1) in the brain tissue of rat fetuses born from pregnant rat PVL models may be related to the function of gelsolin to restore the physiological state through gelsolin-mediated mechanisms. These mechanisms might prevent brain damage following ischemia or reperfusion by controlling inflammation, stabilizing intracellular calcium levels, and breaking down actin polymers generated from injured cells in response to oxygen and glucose shortage (30).
5.1. Conclusions
Using an LPS-established model of PVL, oral piracetam can be employed to prevent CP in fetuses from pregnant rats. The embryonic brain tissue showed decreased levels and expression of GFAP, calmodulin-2 mRNA, and gelsolin mRNA in fetal brain tissue in pregnant rats modeling PVL. Following clinical research, this medication can be recommended for use during pregnancies at risk of premature labor or birth.