1. Background
A chordoma is a type of sarcoma characterized by low-grade, slow-growing behavior, yet it exhibits local invasiveness and aggressiveness (1). The incidence rate varies between 0.18 and 0.84 per million individuals annually and shows geographical and likely racial differences (2). Most chordomas originate in the skull base and sacrococcygeal spine, with a clear male predominance (2). The overall 5-year survival rate is around 50% with en bloc surgical resection followed by proton beam radiation (1).
In the context of skull base chordoma, prior studies have indicated that disease recurrence, incomplete or intralesional tumor resection, absence of postoperative radiotherapy, and the classic chordoma subtype correlate with unfavorable patient outcomes (3-5). Furthermore, studies have provided accumulating evidence supporting the use of molecular biomarkers for predicting prognosis in both skull base and spinal chordomas (5, 6). Recently, the nuclear transcription factor brachyury, an immunohistochemical marker associated with notochordal differentiation, has emerged as an exceptionally sensitive and specific diagnostic tool for identifying chordomas, even in extra-axial locations, and distinguishing them from myoepithelial tumors or para chordomas (7-10).
Brachyury, also known as the T-gene and TBXT, serves as the founding member of a transcription factor family characterized by the presence of the T-box a 200 amino acid DNA-binding domain (11). Located at 6q27 (10), brachyury has recently garnered attention as both a biomarker and a causative factor in chordoma development, making it a promising therapeutic target (12). Notably, a significant subset of brachyury’s targets is involved in cell cycle regulation, including genes such as NUSAP1 and BUB1, which play roles in spindle checkpoint control and cell proliferation (13, 14). Additionally, brachyury influences genes encoding extracellular matrix components, such as laminin alpha2, collagen type VI alpha3, and olfactomedin (15).
2. Objectives
Our team embarked on a novel endeavor to systematically evaluate brachyury’s diagnostic and therapeutic potential in chordomas through a comprehensive review and meta-analysis.
3. Methods
The research question, framed in the context of PECO and PICO, was: What is the prevalence of brachyury expression in chordomas in studies reporting on chordoma patients? Additionally, what are the effects of brachyury therapy on chordomas in studies involving chordoma patients?
3.1. Identification of Articles
A comprehensive review was performed by one researcher (M.S.) across multiple databases, including PubMed/Medline, Scopus, Cochrane Library, and Web of Science, up to July 18, 2024, without any restrictions. Another researcher (A.S.) evaluated the titles and abstracts of the retrieved articles and accessed full texts for those meeting the eligibility criteria. The search strategy involved terms related to chordoma and immunotherapy, including keywords such as ‘immunotherapy,’ ‘immune,’ ‘vaccine,’ ‘brachyury,’ ‘GI-6301,’ and ‘vaccine encoding brachyury.’ Additionally, reference lists of relevant articles were examined to ensure that no significant studies were overlooked. Another author (N.G.) also verified the search and selection procedures.
3.2. Selection Criteria
3.2.1. Inclusion Criteria
(1) Studies examining brachyury expression or brachyury therapy in individuals diagnosed with chordoma.
(2) Chordoma diagnosis is based on either clinical or pathological criteria.
(3) Chordoma patients without other systemic diseases, with control subjects being healthy individuals or those without cancer.
3.2.2. Exclusion Criteria
(1) Review articles, meta-analyses, and systematic reviews.
(2) Studies conducted on animals.
(3) Articles lacking complete data or without a control group.
(4) Conference papers.
(5) Studies where the control group included individuals with systemic diseases.
(6) Book chapters and duplicate studies.
(7) Studies involving cases under active treatment.
3.3. Data Summary
Two authors (M. S. and A. S.) independently collected the data from the original studies. Any differences were resolved through joint discussion.
3.4. Statistical Analyses
The Comprehensive Meta-Analysis version 3.0 (CMA 3.0) software was used to calculate the effect sizes, which were presented as the event rate (ER) with a 95% confidence interval (CI). Begg’s and Egger’s tests were used to assess the presence of publication bias, with P-values less than 0.10 (2-sided) indicating the presence of publication bias.
In terms of sensitivity analysis, the "one-study-removed" and "cumulative" analyses were conducted to assess the disproportionate impact and the effect of each additional study on the overall estimate, respectively. To reduce bias and heterogeneity, we did not include studies with fewer than 10 cases for expression.
We utilized the protein-protein interaction (PPI) network software called STRING to explore functional interactions among the studied genes. Our analysis was conducted using the STRING database (accessed on May 5, 2024; https://string-db.org) with a focus on 'Homo sapiens.' For constructing the PPI networks, we considered interactions with an interaction score exceeding 0.400. In these networks, proteins are represented as nodes, and their interactions are depicted as edges. This approach allowed us to investigate potential interactions among differentially expressed genes related to various tissues.
4. Results
4.1. Study Selection
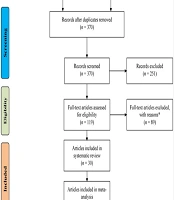
Figure 1 outlines the study selection process for the systematic review or meta-analysis. The initial step involves identifying records from various databases, with a total of 726 records represented in four databases and other electronic sources. After removing duplicates, a total of 370 records remained. Next, the screening phase commenced. All 370 records underwent scrutiny, leading to the exclusion of 251 records due to ineligibility reasons. Subsequently, 119 full-text articles were assessed for eligibility. Among these, 89 articles were excluded based on specific reasons. The eligible studies were then categorized as follows:
- Qualitative synthesis: A total of 30 studies were included.
- Quantitative synthesis (meta-analysis): Another set of 30 studies, including 25 related to the expression of brachyury (7-10, 16-36) and 5 related to the treatment vaccine of brachyury (37-41), made it into this category.
4.2. Study Features
Table 1 shows the characteristics of the studies reporting the expression of brachyury in chordomas. The studies were reported from 2006 to 2024 and included 999 cases of chordoma.
| First Author, Year | Total Cases | Cases with Brachyury Expression | Age (y); (Mean ± SD) | Tumor Site |
|---|---|---|---|---|
| Aviel-Ronen, 2016 (16) | 18 | 18 | 59.2 ± 13.98 | Chest wall, cranium, spine |
| Clayton, 2013 (17) | 10 | 10 | NA | Axial (clivus, cervical spine, thoracic spine, and lumbar spine) and extra-axial (paraspinal soft tissue at L3-L4) |
| Dridi, 2021 (18) | 62 | 37 | 57.7 ± 17.1 | Skull, mobile spine (cervical, thoracic, or lumbar) and the sacrum |
| Hong, 2024 (19) | 100 | 98 | Median (IQR): 44 (30 - 58) | Clivus |
| Indelicato, 2021 (20) | 14 | 13 | median (range): 4.3 (1.0 - 10.7) | Skull base/cervical spine |
| Jäger, 2017 (21) | 24 | 24 | Median (IQR): 49 (40; 80) for clivus versus 70 (51; 75.75) for sacral | Clivus and sacral |
| Jambhekar, 2010 (10) | 51 | 46 | Median (range): 54 (22 - 78) | Axial |
| Jo, 2014 (22) | 20 | 17 | NA | Sacrococcyx, spine, and skull base |
| Kitamura, 2013 (23) | 37 | 30 | 43.6 (range: 10 - 75) | Skull base |
| Lauer, 2013 (24) | 10 | 10 | 44 (range: 13 - 71) | Soft tissue |
| Lv, 2019 (25) | 86 | 86 | 44.8 ± 20.4 | Extra-axial |
| Miettinen, 2015 (26) | 76 | 75 | NA | NA |
| Mobley, 2010 (27) | 14 | 14 | NA | Sacrum and clivus (poorly differentiated chordomas, and typical chordomas) |
| Oakley, 2008 (9) | 73 | 51 | 41 (range: 22 - 62) | Skull base |
| Otani, 2018 (28) | 27 | 27 | Range: 26 - 77 | Skull base |
| Pattankar, 2022 (29) | 20 | 14 | 39.7 ± 13 | Skull base |
| Scheipl, 2012 (30) | 44 | 44 | median (range): 54 (24 - 90) | NA |
| Shen, 2013 (31) | 46 | 46 | Range 17 - 79 | Cords, strands, or solid nests |
| Shih, 2018 (32) | 18 | 18 | 11.9 (range: 1 to 29) | NA |
| Tirabosco, 2008 (7) | 12 | 10 | 45.9 (range: 16 to 68) | Extra-axial skeletal and soft tissue |
| Vujovic, 2006 (8) | 53 | 53 | NA | NA |
| Wakely, 2023 (33) | 24 | 23 | Range: 5 to 81 | Sacrum, hemipelvis, upper back, coccyx, obturator foramen, neural foramen, and vertebra |
| Wang, 2015 (34) | 57 | 52 | 35.7 ± 13 | Skull base |
| Zhai, 2017 (35) | 25 | 25 | 14.44 (range: 8 to 19) | Clivus |
| Zhang, 2013 (36) | 78 | 59 | NA | Sacral and mobile spine |
Characteristics of the Studies Reporting Expression of Brachyury in Chordomas
4.3. Pooled Analysis
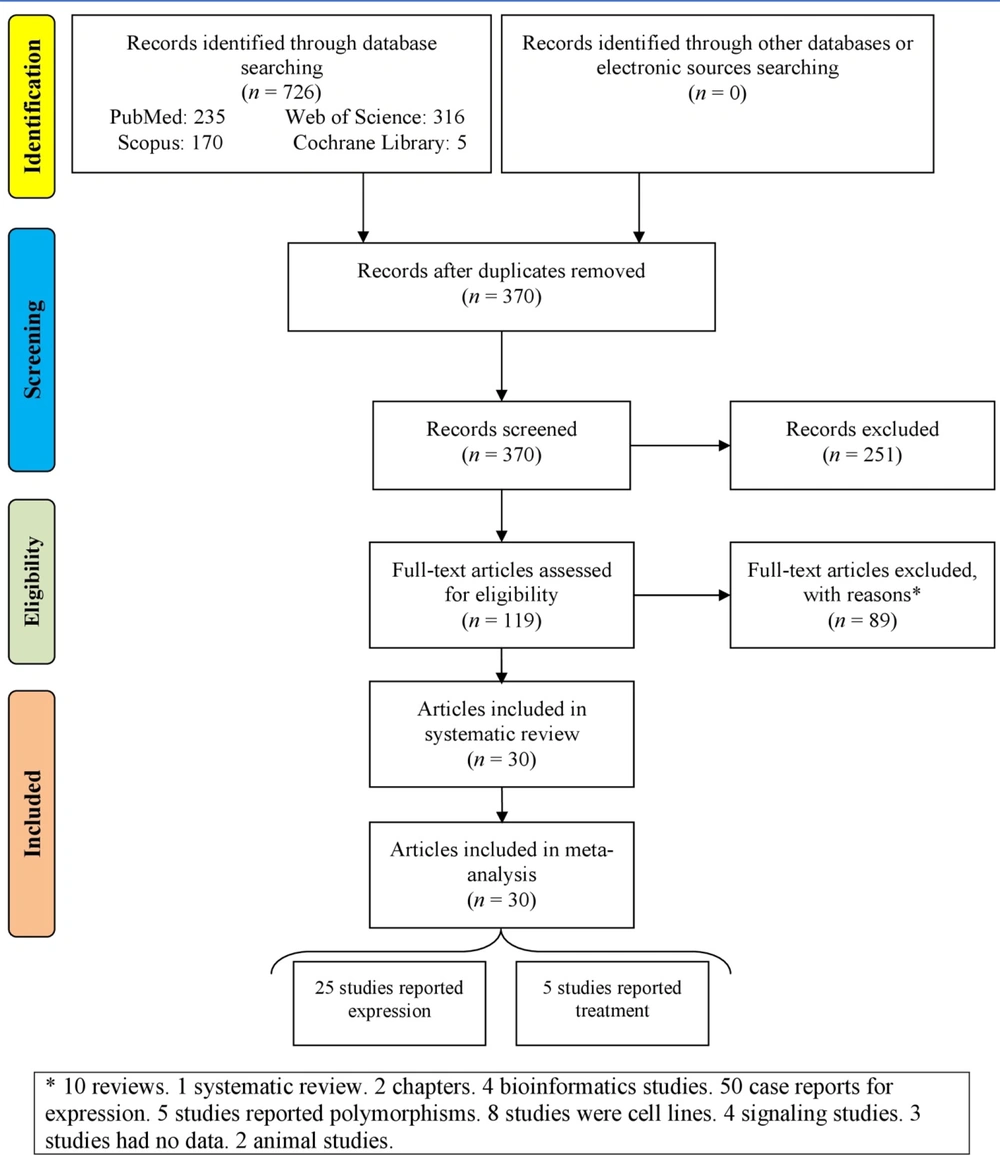
Figure 2 represents the random-effects forest plot of the ER of brachyury expression in chordomas. The ER was 92.2% (95% CI: 87.3% - 95.3%), and I² = 74.65%.
4.4. Sensitivity Analysis
The sensitivity analyses did not change the pooled ER, indicating that the result is stable.
4.5. Publication Bias
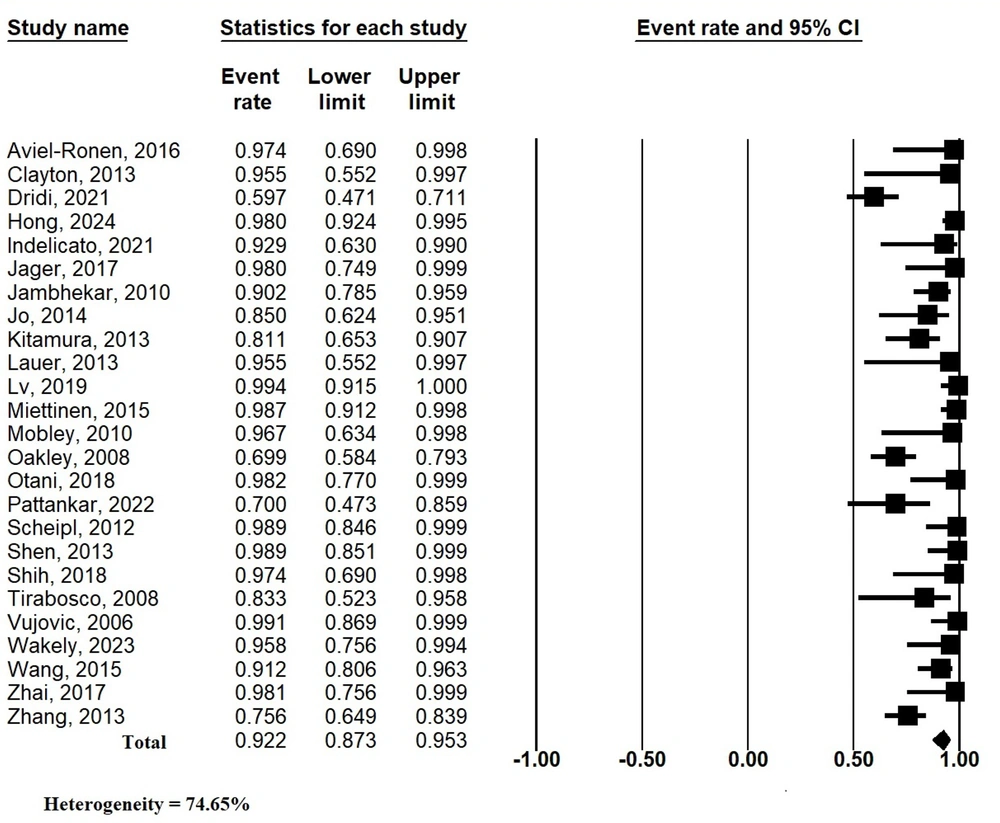
Both Egger's and Begg's tests were performed on the pooled analysis. Figure 3 shows the funnel plot of the ER of brachyury expression in chordomas. The P-values for Egger's and Begg's tests were < 0.00001 and 0.30413, respectively. Therefore, there was moderate bias across the studies.
4.6. Brachyury Therapy
Five studies reported the effect of the brachyury vaccine in chordomas (Table 2). One case report (41), three phase 1 trials (37, 39, 40), and one phase 2 trial (38) reported their results. In a phase 1 trial by Collins et al. (37), the combination of the modified vaccine Ankara (MVA)-Bavarian Nordic-Brachyury vaccine was found to be safe and triggered immune responses against brachyury in advanced chordoma patients. DeMaria et al. (2021) conducted a phase 2 study with the Yeast-Brachyury Vaccine (GI-6301) combined with radiotherapy (RT) in locally advanced chordoma. While GI-6301 was safe, it did not significantly improve the effectiveness of RT (38). DeMaria et al.’s Phase 1 study (2021) using the modified vaccinia Ankara (MVA)-Bavarian Nordic-brachyury TRICOM vaccine showed promising results, including tumor size reduction (39). Heery et al. (2015) explored GI-6301 in advanced chordoma patients, with some disease control observed (40). Lastly, Pastor et al. (2020) reported a case of metastatic chordoma undergoing brachyury vaccine immunotherapy and contracting COVID-19 (41).
| First Author, Year | Study Design | Intervention | Cases | Main Results |
|---|---|---|---|---|
| Collins, 2020 (37) | Phase 1 | Advanced chordoma with modified vaccine Ankara (MVA)-Bavarian Nordic-Brachyury vaccine | 3 | The combination of MVA and fowlpox virus vaccines targeting brachyury is safe and triggers immune responses against brachyury and related antigens, potentially leading to the destruction of tumor cells through immunological mechanisms. |
| DeMaria, 2021 (38) | Phase 2, randomized, double-blind, placebo-controlled | Locally advanced, unresectable chordoma with Yeast-Brachyury Vaccine (GI-6301) combined to Radiotherapy (RT) | 11: GI-6301+ RT group; 13: Placebo group | While GI-6301 was deemed safe, the clinical outcomes and immune data did not demonstrate that the yeast brachyury vaccine improved the in vivo effectiveness of RT in chordoma patients. The median progression-free survival (PFS) for the vaccine and placebo groups was 20.6 and 25.9 months, respectively. Importantly, the vaccine was well tolerated, with no vaccine-related serious adverse events. |
| DeMaria, 2021 a (39) | Phase 1 open-label, 3 + 3 design, dose-escalation | Advanced chordoma with modified vaccinia Ankara (MVA)-Bavarian Nordic-brachyury TRICOM vaccine | 10 | Following treatment, a patient with advanced sacral chordomas exhibited a partial response, which was notable. Additionally, three patients experienced clinical benefits in the form of reduced pain. Notably, there was a dose-dependent trend observed for fever, chills/rigor, and hypotension. Tumor size decreased by 33% and by 66% according to exploratory volumetric measurements. |
| Heery, 2015 (40) | Phase 1 | Advanced chordoma with GI-6301 | 11 | During the trial, two chordoma patients exhibited signs of disease control—one with a mixed response (MR) and another with a partial response. Interestingly, both patients had received radiation treatment for their tumors approximately 3 months before enrolling in the vaccine study. Notably, in the patient with the MR, the lesion that responded to the vaccine was the same one that had previously received radiation, while the non-responding lesion did not. The median progression-free survival (PFS) for patients with chordomas was 253 days (with a range from 41 days to beyond 600 days). |
| Pastor, 2020 (41) | Case report | Metastatic chordoma was diagnosed with COVID-19 while went under intravenous brachyury vaccine immunotherapy | 1 | Brachyury vaccine immunotherapy had a protection against SARS-CoV-2. |
Characteristics of the Studies Reporting the Effect of Brachyury Vaccine in Chordomas
4.7. String Results
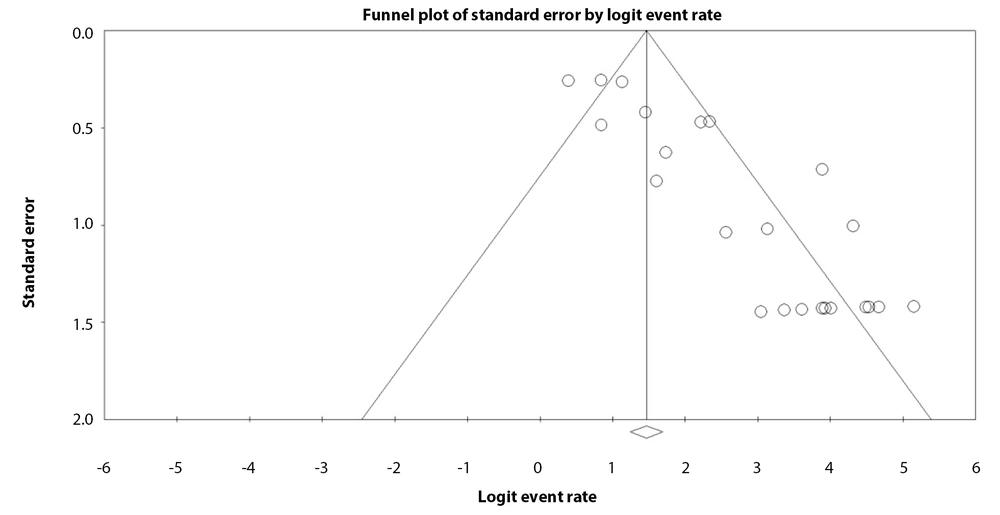
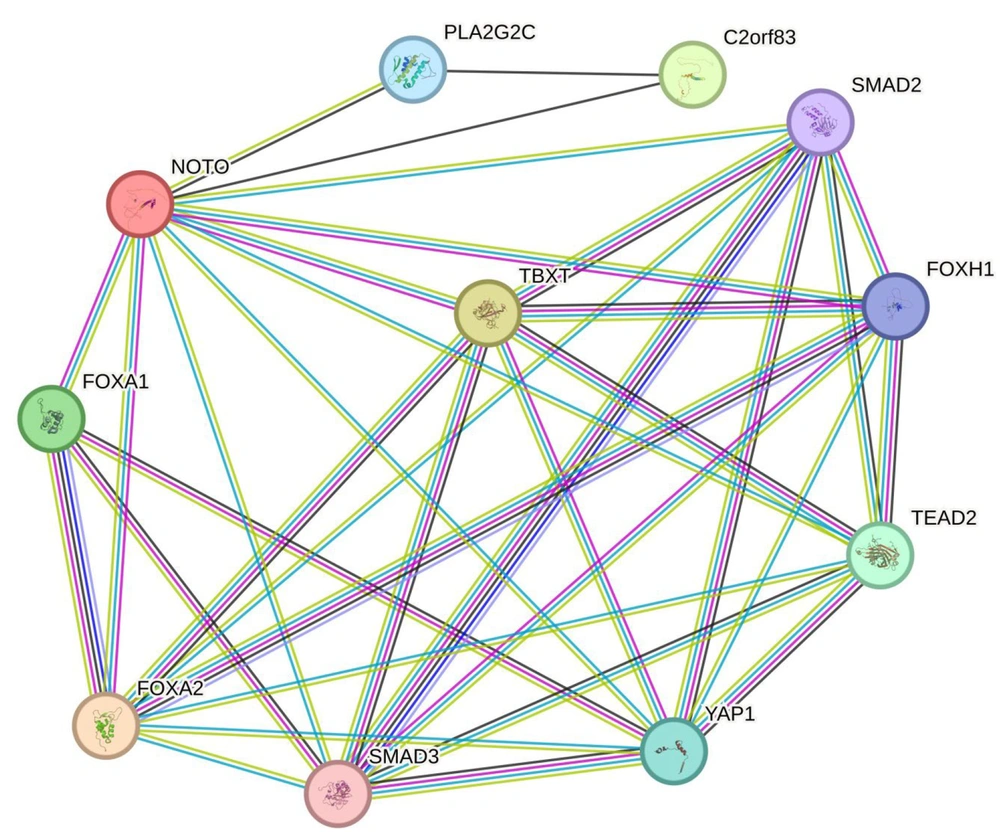
Experimentally determined significant interactions of TBX1 with NOTO, SMAD2, FOXH1, TEAD2, YAP1, SMAD3, and FOXA2 were found in curated databases (Figure 4). This indicates that the significant interaction of TBX1 with YAP1 has been established through experimental and biochemical data. The coexpression analysis revealed only poor coexpression of TBX1 with FOXH1 (coexpression score: 0.103) and FOXA2 (coexpression score: 0.049).
Protein-Protein Interaction (PPI) network graph and heatmap for TBX1 gene from STRING database. The graph shows gene interactions in medium confidence (0.400) involving the number of nodes: 11, number of edges: 35, average of degree: 6.36, PPI enrichment P-value: 9.54e-9. Each node (colored circle) shows a gene, and each edge (line) shows an interaction between two genes.
5. Discussion
An event rate (ER) of 92.2% (95% CI: 87.3% - 95.3%) for brachyury expression in chordomas was observed, with moderate heterogeneity (I² = 74.65%). Publication bias analysis revealed moderate bias across studies. Individual studies explored vaccine safety, clinical outcomes, and tumor response in chordoma patients. Notably, the MVA-Bavarian Nordic-Brachyury vaccine triggered immune responses, but its impact on radiotherapy effectiveness varied. Additionally, a case report suggested that immune-enhancing therapy might offer protection against SARS-CoV-2.
Chordomas, rare malignant tumors occurring along the spine, warrant consideration in the differential diagnosis for midline tumors of the axial skeleton, regardless of their morphology (42). Immunohistochemistry targeting T brachyury can aid in distinguishing chordoma from its mimics (32). Brachyury expression is closely linked to chordoma development and progression (43). Notably, approximately 90% of pathologically confirmed chordomas exhibit positive brachyury expression (9), and our meta-analysis revealed an estimated rate of 92.2% for brachyury expression in chordomas.
Surgical resection remains the established treatment approach for chordoma. However, recent evidence highlights brachyury’s dual role as a diagnostic marker and potential therapeutic target (44, 45). Brachyury is implicated in epithelial-to-mesenchymal transition, along with IL-8 and TGF-β (46-49). These findings suggest that brachyury endows tumor cells with a mesenchymal phenotype, promoting migration and invasion (39, 48). The Bavarian Nordic-Brachyury vaccine, which is well-tolerated and capable of inducing immune responses to brachyury and cascade antigens, shows promising clinical benefits (37-39). Brachyury positivity correlates with improved 5-year progression-free survival in skull base chordoma (29).
The FGF/MEK/ERK pathway influences brachyury expression and signaling in chordomas, potentially contributing to chordomagenesis (45, 50). In mouse embryos, both brachyury and FOXA2 are upstream of the homeobox gene Noto, which is down-regulated in mutant embryos for either gene, suggesting a synergistic interaction within a compact regulatory region (51, 52). Brachyury plays a critical role in stemness regulation in chordoma and other aggressive cancers. Unexpectedly, it controls the synthesis and stability of Yes-associated protein (YAP), a key regulator of tissue growth and homeostasis (53). The brachyury-YAP regulatory pathway is related to tumor aggressiveness, offering potential therapeutic targets (53).
Brachyury has garnered attention in tumorigenesis and therapy due to its activation by multiple signaling pathways and its role in regulating a complex downstream network (48). As clinical trials for therapeutic cancer vaccines continue to develop (37-40), brachyury emerges as a promising target for controlling advanced cancer populations.
Our systematic review and meta-analysis had two significant limitations. First, due to the limited number of trials, we couldn’t thoroughly examine the characteristics and side effects of brachytherapy in chordoma or perform a meta-analysis specifically related to treatment. This limitation arises from the relatively new status of brachytherapy as a treatment for chordoma, leaving room for future trials. Second, there was moderate bias across studies reporting brachyury expression in chordoma. This bias could be attributed to differences in patient selection, including demographic, clinical, and pathological factors.
5.1. Conclusions
An event rate of 92.2% was observed for brachyury expression in chordomas. This high expression suggests that brachyury serves as a reliable diagnostic marker for chordomas. In addition, brachyury serves as a specific marker for chordoma, aiding in its differential diagnosis from other tumors with similar histology. The findings suggest potential therapeutic implications for targeting brachyury in chordoma treatment.
Future research should explore personalized approaches to enhance the efficacy of brachyury-targeted therapies. Investigating the interplay between brachyury expression, immune responses, and treatment outcomes could guide novel therapeutic strategies.