1. Introduction
Psoriasis affects 2 - 4% of the general population and leaves lifelong impressions on these patients (1). The "IL-23/IL-17 pathogenic axis," as the main driving mechanism of psoriasis, involves consecutive activation/maturation of dendritic and T helper cells along with subsequent secretion of inflammatory cytokines, leading to keratinocyte activation and amplification of inflammation (1). Psoriasis vulgaris (white or silvery patches appearing on the "erythematous" skin of affected patients) is the most common type of psoriasis, accounting for 90% of all instances. In this disease, papulosquamous (papule-squamous) plaques are well-distinguished from surrounding normal skin, and the plaques are red or salmon pink in color, coated by white or silvery scales, and may be thick or thin; large or small. Cutaneous lesions most commonly develop on the elbows, knees, scalp, umbilicus, and lumbar regions, and are typically characterized by erythematous plaques covered with silvery‐white scales, termed chronic plaque psoriasis (2, 3).
Multiple synthetic drugs targeting "cytokines, and the relative enzymes, receptors, cell metabolism mediators, cell signaling, and transcription factors, such as tumor necrosis factor-alpha (TNF-α), Interleukin-23 (IL-23), and IL-17," have displayed immense success in the treatment of psoriasis. The first management for patients with mild-to-moderate psoriasis is topical treatment, which is critical for the successful management of the disease (4). Topical steroids (as a standard and customary therapy) (5) have been applied for more than 50 years to treat mild-to-moderate plaque psoriasis. However, therapy success appears to be controversial. Additionally, maintenance periodic therapy appears to be beneficial to lengthen remission while minimizing side effects (6). It appears that, to increase efficacy and minimize side effects, the simultaneous use of steroids and vitamin D analogues or topical retinoids (as pulsed delivery, once weekly, for instance) is the most promising current topical treatment (7). Although with such clear benefits, corticosteroids are accompanied by serious local/systemic side effects, including local cutaneous reactions (atrophy), telangiectases, striae, traumatic purpura, perioral dermatitis, hypertrichosis, rarely contact dermatitis, and adrenal suppression (in widespread use or in infants). These complications become more serious in cases of extensive/widespread lesions (like the case in the current report) and depending on the severity of the disease, which requires more and longer topical/systemic steroid therapy (8).
As stated earlier, the long-term utilization of common systemic therapies for psoriasis is restricted mostly by negligible tolerability and accumulative toxicity. On the other hand, the long-term consumption of biologics is more recommendable due to supreme tolerability and health, although their cost is an issue for sustainability (9). Regarding well-known cutaneous adverse effects (such as atrophy, striae, and/or telangiectases, irritation, phototoxicity) and potential systemic adverse events (carcinogenic immunosuppression), safety concerns, loss of efficacy (or unsatisfied symptom relief), and recurrence after topical drug discontinuation, as well as occasional accessibility concerns of (topical formulations of) biologicals, the scientific community is continuously encouraged to explore novel/effective (natural, specifically plant-derived) topical therapeutic strategies/agents, with minimal adverse side effects.
Flavonoids show protective potential against cutaneous inflammation and photo-aging (10). These bioactive phytochemicals suppress pro-inflammatory mediators/signaling, and due to their anti-inflammatory/antioxidant properties, they exhibit evidence of their capability to prevent skin inflammations like psoriasis. In the research area, there is increasing evidence of flavonoid benefits in managing psoriasis, targeting both immune cells and keratinocytes (11). Quercetin, luteolin, delphinidin, and baicalein have shown significant antipsoriatic effects (11).
The bioavailability of flavonoids depends on both physicochemical and pharmacokinetic factors (including the chemical structure, polarity, molecular mass, sensitivity to pH and gastrointestinal enzymes, and degree of intestinal absorption). After absorption and subsequent metabolic transformation in the small intestine, liver, and colon, the conjugated aglycones are often devoid of the pronounced biological activity characteristic of the unmodified flavonoids. Topical application of flavonoids (mainly as aglycone) is also accompanied by similar challenges when it is necessary to overcome the skin barrier and guarantee their bioavailability to the target cells, a fundamentally important factor in enhancing their biological action (12).
Regarding the structure–permeation relationship, flavonoids reveal somewhat different physicochemical characteristics based on the different types and numbers of functional moieties. The physicochemical properties of these natural penetrants influence their cutaneous absorption/skin delivery or targeting (10, 13-15). Although, based on the LogP values, flavonoids are classified as relatively lipophilic compounds, the planar structure, specifically in the flavonols and flavones, as well as the limited flexibility of the chiral center in the C ring, make “skin penetration of flavonoids” a serious challenge. Thus, flavonoids with fewer hydrogen bonds, total polarity surface, and molecular volume are advantageous for facile skin absorption (13).
Flavonols (such as quercetin, with numerous reported health benefits) are distributed ubiquitously among different plant foods. However, its HO- functional groups (its hydrophilicity) can compromise its use in lipophilic in vivo systems. Hence, many endeavors have been conducted to maximize its yield by various means, including structure amendment. It is also expected that the effectiveness of flavonoids will increase following the increase in compound lipophilicity (by reversible acylation of flavonoids). Prodrug contraption is a highly recognized molecular amendment strategy that aims to optimize the physicochemical and pharmacological traits of drugs to improve their solubility and pharmacokinetic characteristics and reduce their toxicity. In vivo (intracellular) bio-activation of prodrugs, by enzymatic and/or chemical transformations, creates a biologically active drug (the aglycone flavonoid) and a safe promoiety (e.g., organic acids) (16). In this strategy, the quercetin precursor (or quercetin ester derivative), exhibiting resistance to phase II metabolism, might provide useful means to increase the level of systemic (or intracellular) aglycone and increase its bioavailability when the quercetin prodrug undergoes partial/full deacylation (17).
In the current case report, inspired by (18) and other literature, the aim of the current project was the development of a bioavailability-enhancing precursor of quercetin followed by its application for the successful/safe treatment of a “psoriasis vulgaris” case. Moreover, since quick/easy elimination of residual promoiety (acyl) groups is expected to take place in vivo, suitable precursors of other biologically active natural products may constitute a useful method to increase systemic/intracellular active substance (aglycone flavonoid, in the current study) concentrations. In the current study, we report a 36-year-old married woman who presented with widespread psoriasis on the face, as well as some parts of the upper/lower extremities (Figure 1). Also, with a brief review of the literature, we report herein a successful therapeutic approach using acyl-quercetin (InflAQ). The employed topical intervention led to the complete regression of the severe disease. Therefore, although it may be classified as an unconventional/non-standard modality (5), we consider/propose topical InflAQ as promising (and safe, as well) for the treatment of psoriasis vulgaris. This individual decision model (19) takes into consideration patients’ preferences and previous treatment experiences.
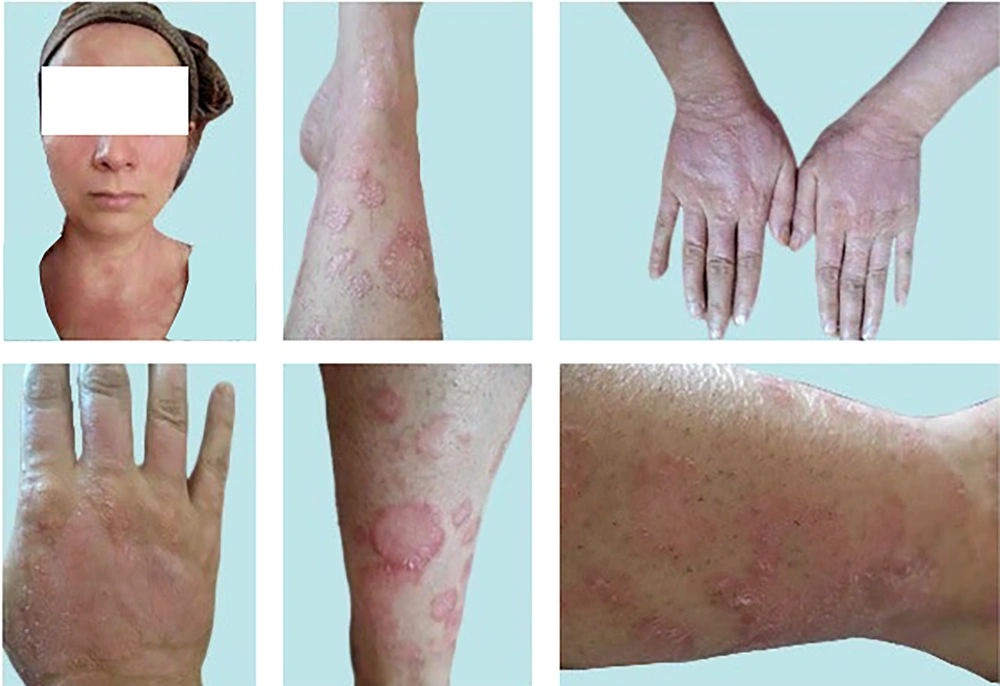
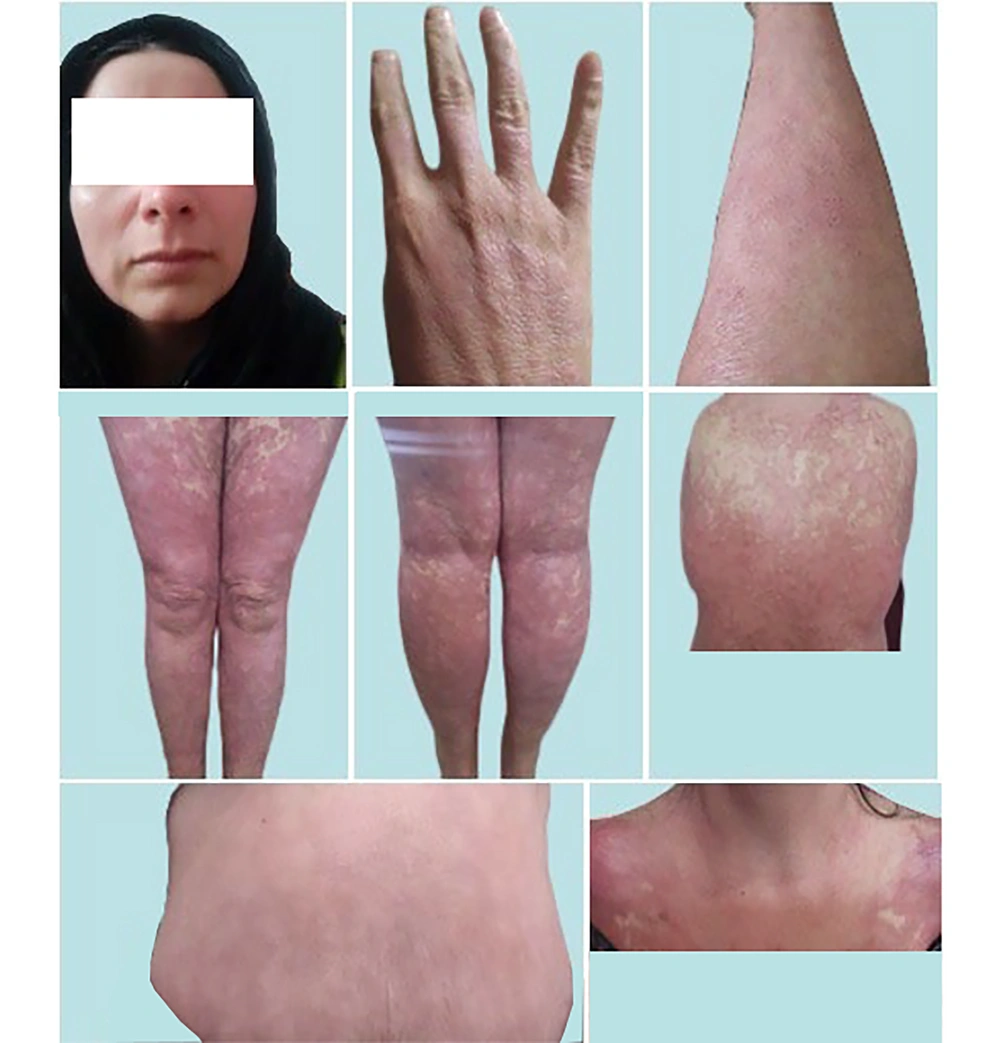
Pre-treatment skin images of face/neck lesions (top, left image), and lower/upper extremities (other images). In that time (early July, 2023) and long before the intervention with InflAQ, the patient was only using vaseline/petroleum jelly (twice a day) as an emollient and had absolutely preferred not to use any usual disease-modifying topical treatment. She accepted this type of intervention (InflAQ included in cream base, USP/BP), which is only based on the natural compound. Her daily living activities were severely affected (for several years) by the burden of widespread psoriatic skin lesions (only in face/neck/extremities, almost with no itching).
2. Case Presentation and Discussion
A 36-year-old married woman presented with a history of approximately 26 years of redness/erythema, thickness/induration, and scaling/desquamation of widespread psoriatic skin lesions, diagnosed as psoriasis vulgaris, affecting her face/neck and upper/lower extremities (Figure 1). Upon admission, the affected skin displayed large areas of raised, well-demarcated, erythematous plaques with adherent silvery scales, and pustules developed on erythematous areas. Interestingly, she did not report a burning sensation or irritating itching on her body but experienced severe discomfort during daily activities and sleep. According to the patient, the first psoriasis lesion appeared on her left knee at around age 10 and then spread to other parts of her body, severely affecting her daily living activities for several years.
At the time she accepted the intervention (early July 2023) and long before receiving InflAQ, the patient was only using Vaseline/Petroleum jelly (twice a day) as an emollient and had chosen not to use any usual disease-modifying topical treatments like steroids. She continued using the emollient throughout the study period on the advice of the dermatologist (the first author, A. E.). Over the past many years, the patient had tried homeopathy (20), topical corticosteroids, and a vegetarian regimen for treatment, without any history of using topical natural products or traditional medicines. All the mentioned therapies either did not provide sufficient relief of psoriatic lesions or were abandoned due to concerns about side effects from long-term use. Approximately 15 years ago, she experienced widespread erythrodermic psoriasis (EP) secondary to psoriasis vulgaris (21) after using topical steroids. Following this, and according to the patient’s declarations, she gradually stopped using topical steroids completely, and after a long period accompanied by only moisturizing treatment, she overcame the EP, and the lesions were again limited to her face and extremities.
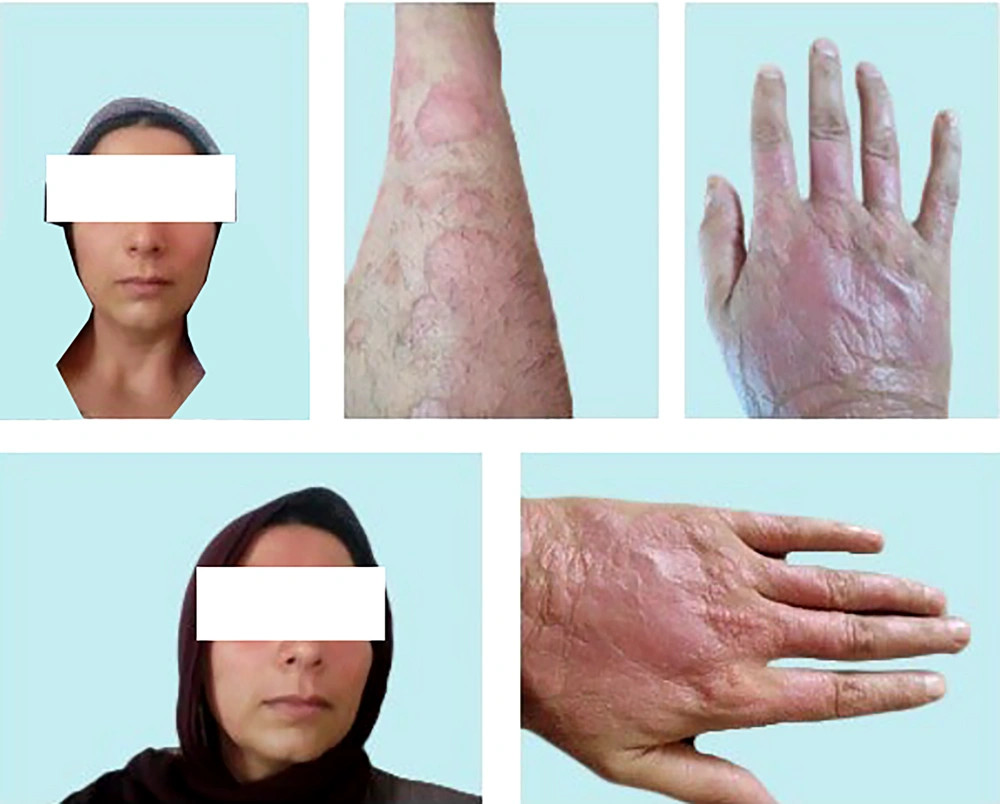
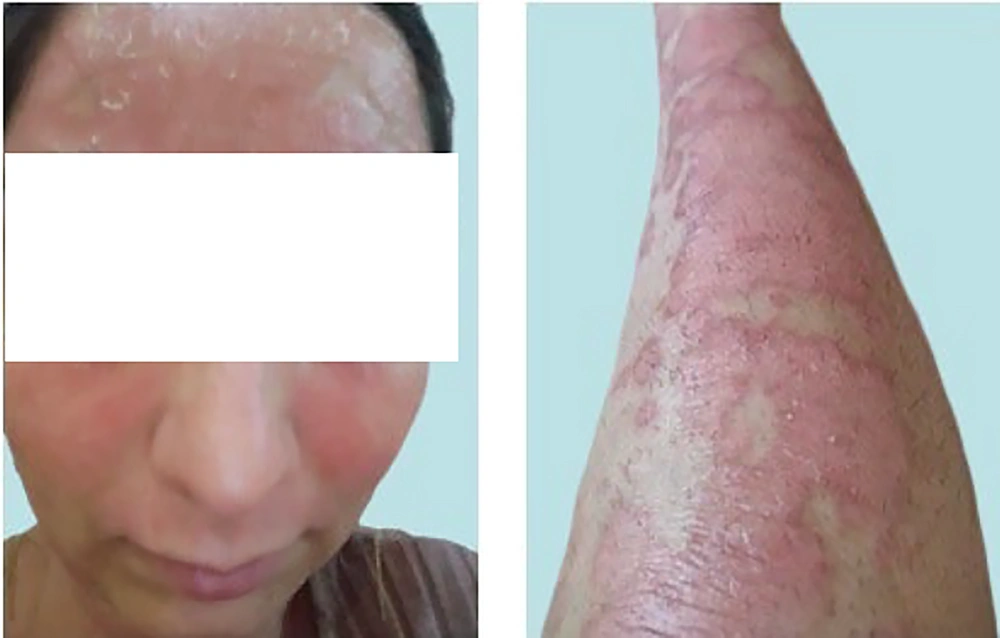
The modified quercetin (InflAQ) was synthesized once every month, purified, and formulated as a cream (2 mg/mL) using a commercial/approved cream base (www.sepidaj.com) and stored in a refrigerator (4°C). As indicated in Figure 2, one month after starting treatment with InflAQ monotherapy (late July 2023), a remarkable improvement in clinical appearance was achieved, specifically on the face and lower extremities. However, the lesions on the hands remained almost unaffected compared to the pretreatment skin images in Figure 1. About two months later (late August 2023), according to Figure 3, all variables, including erythema, scaling, and thickness, were substantially reduced in areas where the formulated InflAQ cream was applied. This was considered the first sign of the effectiveness of plant-derived InflAQ monotherapy in controlling the inflammatory burden on the psoriatic patient (22).
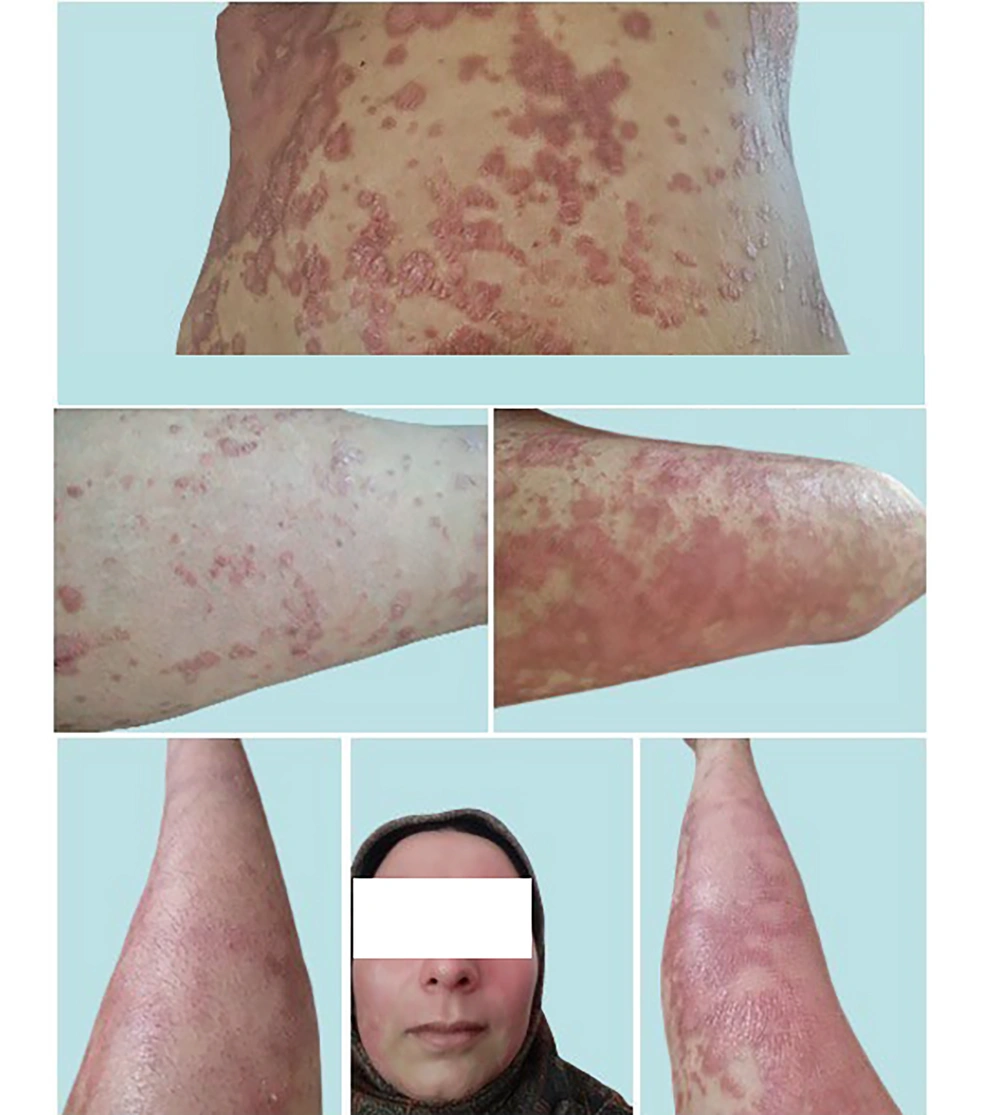
Unexpectedly, between September 10 - 30, 2023, widespread erythrodermic psoriasis (EP) symptoms first appeared on the patient's abdominal skin and then on the anterior lower extremities as new lesions (Figure 4). A widespread redness/inflammation appeared suddenly over a couple of days and was considered erythroderma following or secondary to psoriasis vulgaris. The patient insisted that she did not stop or over-apply topical treatments. Erythrodermic psoriasis is clinically defined as prominent erythema and scaling affecting at least 75 - 90% of the body surface area (BSA) (23). Due to extensive cutaneous involvement, EP patients can present with systemic symptoms such as pruritus, fever, chills, dehydration, arthralgia, asthenia, and lymphadenopathy (24). In this EP case, the patient only occasionally complained of mild chills with no other symptoms. Moreover, lesions on the face and hands showed promising improvements (Figure 4).
It is noteworthy that the severity of psoriasis was assessed with the Psoriasis Area and Severity Index (PASI), which combines the intensity of redness/erythema, thickness/induration, and scaling/desquamation of psoriasis. The severity of psoriasis according to the PASI was defined as mild (PASI < 7), moderate (PASI 7 - 12), and severe (PASI > 12) (24). Exceptions are defined as so-called “upgrade criteria”; if visible (e.g., on the face, scalp, hands, or nails) and/or therapy-refractory regions are affected, psoriasis can be assessed as moderate-to-severe, even if the affected BSA is 10. The above statements and the patient status (EP) (23) were indications for systemic treatment. However, despite showing indications for systemic treatment between September and October 2023 (Figures 4 - 5), the patient refused systemic treatment and insisted on continuing with this type of topical treatment. Therefore, considering the satisfactory health status and the patient’s expectations, we decided to continue with InflAQ topical treatment for both old and newly developed EP lesions.
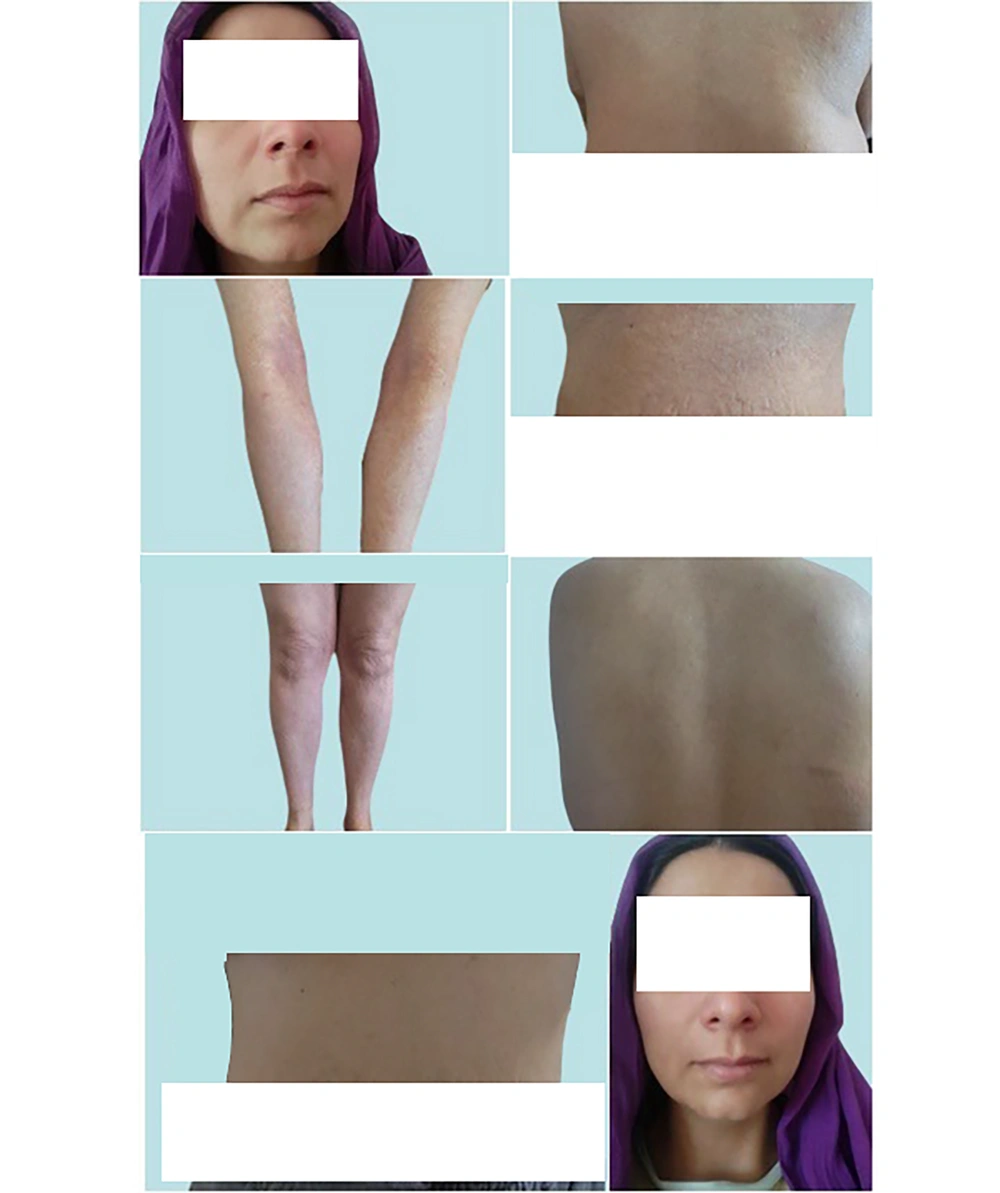
A few months after starting treatment with InflAQ (October 20, 2023), a remarkable normalization of the clinical appearance on the face, hand, and secondary abdominal EP lesions was achieved. However, as evidenced in Figure 5, widespread secondary erythema appeared again on the posterior skin and anterior/posterior lower extremities. InflAQ topical treatment was also applied to these areas. At this time, during the maintenance phase, the patient continued with the same therapeutic agent (InflAQ) at a reduced frequency for the relatively healed abdominal, face, foot, and hand skin. In November 2023 and afterward, following InflAQ treatment, the patient experienced complete relief and showed significant improvement in the primary/EP skin lesions (Figure 6), achieving remarkable normalization of the skin.
Significant research, including the current study, highlights the therapeutic benefits of flavonoids in various skin conditions, as these compounds have been shown to absorb ultraviolet radiation and modulate inflammation-related signaling pathways (25). Moreover, the structures of flavonoids are compatible with the roles of inhibitors or substrates of the tyrosinase enzyme, highlighting their importance in the cosmetic industry (26). Limited skin penetrance and poor stability are two major limitations. Flavanones, the best penetrant flavonoids, were found to mitigate some features of psoriasis, such as scaling and epidermal hyperplasia; however, it cannot be claimed that these compounds, among other flavonoids, are the strongest modulators (13). An in vivo study demonstrated that the levels of pro-inflammatory factors (TNF-α, IL-6, and IL-17) were significantly decreased after oral administration of different doses of quercetin in an imiquimod-induced psoriasis-like skin inflammation in mice (27). Although the prospects for the application of nanocarriers for targeted delivery of flavonoids with low bioavailability are beyond doubt, the promising flavonoid delivery systems have mostly been reproduced in vitro and, to a lesser extent, in animal models. In a human study, the antipsoriatic activity of quercetin as a spanlastic formulation increased greatly, suggesting that spanlastics can be a promising, easily prepared formulation to overcome poor aqueous solubility and penetrability of flavonoids for the efficient treatment of psoriasis (28, 29). Again, flavonoids, with minimal safety concerns, have been employed in clinical trials against human skin diseases. Administration of a quercetin-containing hydrogel, used in a clinical trial to treat lower extremity skin wounds in diabetic patients, led to complete wound healing in 9 out of 58 patients and health improvement in the rest (30). Since the 3-OH and 7-OH hydroxyl groups of quercetin are involved in oxidative degradation and metabolism, respectively, and since oxidative degradation, mediated or facilitated by intracellular pro-oxidative compounds such as enzymes and transition metal cations, has been reported as the main cause of rapid elimination of quercetin, we set out to transiently protect those hydroxyl groups with a metabolically susceptible promoiety. This would provide safe cell delivery followed by the slow release of quercetin in the intracellular compartment, resulting in a sustained therapeutic effect against psoriasis vulgaris (31, 32).
3. Conclusions
We present a case of a female patient suffering from psoriasis vulgaris, also known as chronic stationary psoriasis or plaque-like psoriasis, who was successfully treated using a modified version of quercetin, referred to as pro-drug/quercetin. In light of the above statements, we, as part of the scientific community, are convinced that more comprehensive clinical trials conducted in the near future will greatly contribute to improving the effectiveness and safety of new treatment methods for inflammatory skin diseases such as psoriasis.