1. Background
Multiple sclerosis (MS) is the most common chronic inflammatory disease in the central nervous system (CNS). There are approximately 3 million MS patients worldwide, although the prevalence varies but is increasing in different countries (1). In this disease, the brain or spinal cord may be affected. Multiple sclerosis typically occurs between the ages of 20 - 30 years. It can affect physical functioning, cognitive function, quality of life, and even employment of patients, causing severe damage to their health and well-being (2). The most common phenotype of MS is relapsing-remitting multiple sclerosis (RRMS) (3).
Multiple sclerosis is associated with many manifestations and functional disorders, including cognitive impairment. Disorders in cognition are catastrophic because it has a relatively high prevalence and affects all aspects of the lives of such young patients. About 40 - 65% of MS patients suffer from cognitive impairment, and the highest was related to information processing (32%) (4, 5). In some patients, cognitive impairment can be the first manifestation of the disease (4, 6-8). In the past three decades, the diagnosis of cognitive impairment in MS patients has greatly increased. It seems that cognitive impairment is an influential part of the clinical manifestations of patients and has devastating effects on an individual's autonomy and daily functioning. Unfortunately, despite the high prevalence and harmful effects of cognitive impairment, the effective treatments for this aspect of the disease are negligible (9).
Levetiracetam (LEV), the S-enantiomer of α-ethyl-2-oxo-1-pyrrolidine acetamide, is an antiepileptic drug. The three main mechanisms of action known for the drug LEV include binding to the synaptic vesicle 2A (SV2A), which also plays a role in the inhibition of calcium channels (N typing), and the moderating effect of this drug on gamma amino butyric acid (GABA) receptor. Also, the neuroprotective effects of LEV are a new area of research about this medication, although no specific mechanism has yet been found (10). Levetiracetam is indicated for the treatment of partial-onset seizures, adjunctive therapy for the treatment of myoclonic seizures, and primary generalized tonic-clonic seizure (11).
The role of SV2A and synaptic vesicle 2B (SV2B) in the entry of amyloid beta into the synaptic terminal is unclear. But it seems that it plays a role in the incidence of symptoms of cognitive impairment in patients with Alzheimer's disease (12).
A 2013 study on the effect of LEV on behavioral changes, neuropsychology, and electroencephalogram (EEG) of healthy people showed that LEV improves managerial performance in these subjects (13). In another study, LEV had beneficial effects on speech memory in patients with high-grade glioma (14).
A review of the effect of LEV on intracerebral hemorrhage (ICH) patients shows that patients with ICH treated with LEV had better cognition levels when discharged from the hospital (15).
Also, long-term treatment with LEV prevents remodeling, behavioral disorders, synaptic dysfunction, and learning and memory problems in human amyloid precursor protein (HAPP) mice (16).
Levetiracetam ultimately reduces neurotransmitter release and prevents neural message transmission in the synaptic cleft. Glutamate is a key neurotransmitter in many aspects of brain function, such as cognition, memory, and learning, and impaired glutamate homeostasis will be associated with neurological consequences and appears to be a common factor in the pathogenesis of CNS diseases such as Alzheimer's and Parkinson's disease, concussion, schizophrenia, epilepsy, and MS (17). Glutamate is the most abundant excitatory neurotransmitter in the CNS. Under normal physiological conditions, the main source of CNS glutamate outside the cell is pre-synaptic neurons. Glutamate is activated after release by pre-synaptic and post-synaptic nerve cells and glial cells. Glutamate concentrations should be regulated by endogenous mechanisms, and glutamate signaling disorder is associated with cognitive dysfunction. Increasing the concentration of glutamate in synaptic space leads to apoptosis stimulation. It leads to brain lesions and cell necrosis, called glutamate excitotoxicity, which plays a role in this cell necrosis, delayed apoptosis of calcium-dependent pathways, or sodium ion influx ion. Various damages in the brain can cause glutamate excitotoxicity (18).
A study done by Solaro et al. looked at how well LEV works for improving arm movement in people with MS who have problems with their cerebellum. This study was done in many places and used a method where some people got a fake treatment to compare with the real one. The results showed that LEV helps make arm movements better, as seen in a test called the nine-hole peg test (9HPT), and also improves overall arm function in these patients (19).
In a retrospective study on the effects of LEV in treating spasticity, LEV was effective on phasic spasticity (20).
In a randomized, double-blind trial for neuropathic pain in MS, LEV was compared to a placebo for six weeks at a dose of 3000 mg per day. Levetiracetam was only effective in patients with MS with specific pain symptoms. Also, in central neuropathic pain of patients with MS, the administration of LEV for three months showed a significant difference in pain control and improvement of the patient's quality of life (21, 22).
2. Objectives
Considering the mechanisms mentioned for LEV and the results it has had in similar diseases, as well as due to the high prevalence of MS and the importance of cognitive disorders in this disease and lack of effective treatment, we in this study focused on the effect of LEV on cognitive disorders in patients with RRMS based on the minimal assessment of cognitive function in multiple sclerosis (MACFIMS) test.
3. Methods
3.1. Study Design and Setting
This study was a pilot randomized (23), double-blinded, placebo-controlled trial conducted from December 2021 to November 2022 in patients referred to Sina Hospital's MS Research Center and Neurology Clinic affiliated with TUMS. This study protocol was approved by the Ethics Committee of Tehran University of Medical Sciences (approval date: 2021-08-02, ethics committee: IR.TUMS.MEDICINE.REC.1400.515). The study was registered in the Iranian Registry of Clinical Trials (IRCT registration information: IRCT20210707051810N2, Registration date: 2021-10-05).
3.2. Participants
Patients aged 18 - 60 years with a definitive diagnosis of RRMS, according to modified McDonald's 2017 criteria, were enrolled in the study (24).
Inclusion criteria were as follows: (1) Expanded Disability Status Scale (EDSS) smaller than or equal to 5.5; (2) treatment with injectable or oral first-line drugs (interferon beta, glatiramer acetate, dimethyl fumarate, and teriflunomide); (3) no change in type and dosage of disease-modulating drugs in the last six months; (4) passing of at least two years from a definite diagnosis of RRMS; (5) not receiving corticosteroids in the past 30 days; (6) not experiencing an attack in the past sixty days (25).
Exclusion criteria included: (1) Pregnancy and lactation, (2) seizure disorder, (3) history of any suicide attempt and a history of drug or alcohol abuse in the last six months, (4) uncontrolled acute or major mental disorder that can affect patients' cognitive function, such as depression, schizophrenia, anxiety, etc. (if the therapeutic regimen was the same during the past three months, the disease was assumed as controlled); (5) chronic kidney disease (eGFR ≤ 60 mL/min) (26); (6) diabetes mellitus; (7) hypothyroidism (27); (8) anemia (28); (9) developing any MS attack during the study period; (10) concomitant use of drugs that may affect cognitive function, such as antipsychotics, modafinil, methylphenidate, amphetamine, and amphetamine-like compounds, tricyclic antidepressants, and anticonvulsants other than gabapentin and pregabalin, benzodiazepines other than as sleeping pills (if the dose and method of medication do not change in the last three months and during the study, this will not be prohibited), Incidence of each of the above disorders during the study.
After explaining the objectives and methods of conducting the study, if the patient agreed to participate and completed the consent form, they were enrolled in the study.
3.3. Study Protocol
The patients were divided into two groups using the randomization of a quadrupled block (Permuted block randomization). The random sequence was generated by an epidemiologist using the online program and ensured through the block randomization method. Patients in the intervention group were treated with LEV (Levebel 500 mg, produced by Cobel Darou Pharmaceutical Company), while patients in the control group received a placebo. The appearance and color of the original drug and placebo were identical (similar matching placebo) and were prepared by the Pharmaceutical Company.
The initial dose was 250 mg (half a pill) administered twice a day, and 500 mg was added weekly to the total daily dose until the dose reached 1000 mg twice a day (2000 mg per day). The placebo was tapered in the same way as LEV.
All participants, the principal investigator, and the healthcare personnel responsible for patient care were blinded to the allocation of the original drug or placebo. To maintain blinding, the drug and placebo were packed into groups A and B by a third party who had no involvement in the selection or delivery of the medication. Other treatment strategies for MS or any other conditions were continued under the supervision of a neurologist or the relevant specialist. These treatments were recorded in detail during the study and follow-up visits.
The pill-counting method was used to assess the patient’s compliance with the drug regimen during the intervention, and adherence was confirmed to be over 80%. Throughout the study, all patients were contacted via telephone every two weeks to ensure adherence to treatment and to monitor for possible adverse effects or complications associated with the use of LEV (29).
3.4. Instruments and Data Collection
The MACFIMS was accomplished by seven tests: (1) California verbal learning test-II (CVLT-II), (2) paced auditory serial addition test (PASAT), (3) symbol digit modalities testing (SDMT), (4) brief visuospatial memory test-revised (BVMT-R), (5) delis-Kaplan executive function system (D-KEFS), (6) controlled oral word association test (COWAT), and (7) judgment of line orientation (JLO). Therefore, five parts of cognition, including information processing speed, managerial performance, attention, memory, and spatial perception, were evaluated. The PASAT test was the first scale used in the MACFIMS test, which is working memory to evaluate the speed of information processing, disruption of temporary storage, and simultaneous processing of visual and verbal information (30). The second SDMT scale measures the speed of information processing and working memory measurement (31). California verbal learning test-II is approved as a good scale for memory and learning evaluation. At this stage, the aim is to measure the subject's free learning ability, which is impaired in most MS patients (32). Another subtest includes BVMT-R, one of the MACFIMS test scales, used to measure the spatial-visual performance and memory of MS patients (33). Another test consists of the classification of D-KEFS cards, which is abstract for evaluating reasoning and can distinguish conceptual thinking and intellectual flexibility. Another cognitive disorder in MS patients is visual-spatial abilities disorder, which the JLO test has been approved as the best tool to value this function. The COWAT test is used to accurately measure and assess language dysfunctions to assess the verbal fluid (34, 35). The MACFIMS test was administered by an expert in the implementation of the test at the beginning and after 16 weeks for patients based on validity and reliability tests of the Persian translation of the minimal assessment of cognitive function in MS (36).
All information, including demographics, past medical history, past drug history, and current and past MS medical programs, was recorded. At the beginning and the end of the 16th week, the MACFIMS test was performed to evaluate the cognitive function of patients.
In this study, patients were also assessed for possible complications of LEV using the Neuropsychiatric Inventory (NPI) Questionnaire at the 4th and 16th weeks. The NPI is an information-based interview designed to evaluate neuropsychiatric symptoms in patients with neurodegenerative disorders such as Alzheimer's disease (37, 38). This test assesses ten behavioral domains: (1) Delusions, (2) hallucinations, (3) agitation, (4) depression, (5) euphoria, (6) anxiety, (7) indifference, (8) disinhibition, (9) irritability, (10) abnormal motor behaviors, (11) behavioral evaluation includes the frequency and severity of each behavior. The final score for each section is calculated by multiplying the intensity score by the number of occurrences of the behavior (39).
3.5. Statistical Analysis
All analyses were conducted using the statistical package for the social sciences (SPSS) for Windows, version 26.0. A level of P < 0.05 was considered statistically significant. The primary outcome measure of effectiveness was the change in cognitive domains based on the MACFIMS compared to baseline. The MACFIMS was administered using seven tests: CVLT-II, PASAT, SDMT, BVMT-R, D-KEFS, COWAT, and JLO, evaluating five cognitive domains: Information processing speed, managerial performance, attention, memory, spatial perception, Secondary outcomes included side effects.
4. Results
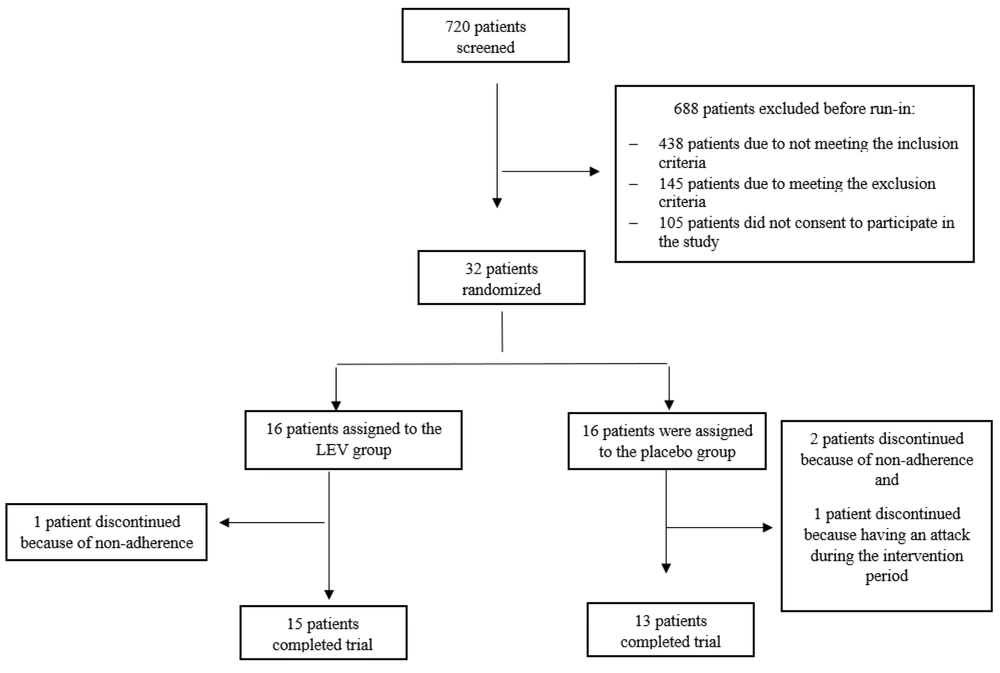
In total, 32 RRMS patients met the inclusion criteria and were recruited into the study. These patients were randomly assigned to two study groups, with 16 participants in each group. As shown in the participants' diagram, one patient in the LEV group and three in the placebo group dropped out due to non-adherence and experiencing an MS attack during the intervention period.
Finally, 15 patients in the LEV group and 13 patients in the placebo group completed the study and were included in the analysis (Figure 1).
Table 1 presents the baseline characteristics of participants in each group. There were no significant differences in demographics or clinical characteristics between the two study groups (P > 0.05).
| Variables | Intervention Group | Placebo Group | P-Value |
|---|---|---|---|
| Age (y) | 46.2 ± 8.1 | 41.31 ± 7.27 | 0.10 |
| Duration of disease (y) | 10.7 ± 6.5 | 7.3 ± 4.0 | 0.11 |
| Female gender | 14 (93.3) | 13 (100) | 0.34 |
| Educational level | 0.36 | ||
| Illiterate | 0 (0) | 0 (0) | |
| Primary school | 1 (6.7) | 2 (15.4) | |
| Guidance school | 1 (6.7) | 1 (7.7) | |
| Diploma | 6 (40) | 2 (15.4) | |
| Associated degree | 2 (13.3) | 0 (0) | |
| Bachelor degree | 3 (20.0) | 6 (46.2) | |
| Master’s degree | 2 (13.3) | 1 (7.7) | |
| PhD | 0 (0) | 1 (7.7) | |
| MS treatment drug | 0.37 | ||
| Dimethyl fumarate | 5 (33.3) | 4 (30.8) | |
| Interferon beta | 7 (46.7) | 3 (23.1) | |
| Teriflunomide | 0 (0) | 1 (7.7) | |
| Glatiramer acetate | 3 (20.0) | 5 (38.5) |
The mean ± SD age of participants was 46.2 ± 8.1 years in the LEV group and 41.31 ± 7.27 years in the placebo group (P = 0.10). Fourteen participants (93.3%) in the intervention group and all participants in the placebo group were female (P = 0.34). Most participants in the LEV group were receiving Interferon beta (n = 7, 46.7%), while the majority in the placebo group were receiving Glatiramer acetate (n = 5, 38.5%) for MS treatment (P = 0.37).
The adverse reactions to the study interventions are shown in Table 2, too. Ten (76.9%) patients in the placebo group and 12 (80%) patients in the LEV group reported no adverse reactions. The most symptoms recorded for LEV were gastrointestinal (n = 2) 13.3%, and CNS (ataxia, dizziness) 6.7% prevalence (n = 1). The placebo group reported gastrointestinal reactions and CNS-related adverse effects (dizziness) and sleep disorders by 7.7% prevalence (n = 1). Psychological complications based on the NPI test were not reported in any patients in the drug and placebo group who had completed the treatment period.
| Variable | Intervention Group | Placebo Group | P-Value |
|---|---|---|---|
| Adverse drug reactions | 0.15 | ||
| None | 12 (80) | 10 (76.9) | |
| CNS (ataxia, dizziness) | 1 (6.7) | 1 (7.7) | |
| Gastrointestinal | 2 (13.3) | 1 (7.7) | |
| Sleep disorders | 0 (0) | 1 (7.7) |
The Distribution of Adverse Drug Reaction Related to Levetiracetam and Placebo in Patient Included in the Study
The comparisons of MACFIMS scores at the baseline and changes in MACFIMS scores between LEV and placebo groups are mentioned in Table 3. There was no significant difference in the case of the ten MACFIMS subtests between the two study groups (P > 0.05). The change in JLO score in the LEV group was significantly greater than in the placebo group (3.80 ± 4.10 vs. 0.23 ± 4.36; P = 0.03). The number of changes observed in the CVLT-II, BVMT-R, PASAT, COWAT, and BVMT-R-delay subtests in the intervention group was increasing, while these changes in the placebo group had a decreasing trend. While the differences in these changes were not statistically significant between the two study groups, it is valuable from a clinical point of view.
| Variables | LEV | Placebo | P-Value a |
|---|---|---|---|
| MACFIMS scores at the base of the study | |||
| CVLT-II | 46.20 ± 13.41 | 50.92 ± 15.24 | 0.39 |
| SDMT | 42.20 ± 15.60 | 48.46 ± 17.55 | 0.32 |
| BVMT-R | 17.33 ± 8.06 | 23.30 ± 10.14 | 0.09 |
| PASAT | 28.87 ± 22.15 | 35.15 ± 20.72 | 0.58 |
| COWAT | 30.86 ± 12.14 | 28.46 ± 8.96 | 0.56 |
| DKEFS-descriptive | 6.47 ± 2.26 | 6.54 ± 2.03 | 0.96 |
| DKEFS-sorting | 25.07 ± 9.85 | 25.23 ± 7.51 | 0.96 |
| JLO | 17.93 ± 4.21 | 20.69 ± 5.29 | 0.13 |
| CVLT-II-delay | 13.13 ± 12.79 | 11.15 ± 2.51 | 0.58 |
| BVMT-R-delay | 7.40 ± 4.06 | 9.85 ± 3.08 | 0.07 |
| Change of MACFIMS scores | |||
| CVLT-II | 3.26 ± 14.43 | -6.30 ± 11.66 | 0.06 |
| SDMT | 10.13 ± 14.12 | 4.00 ± 4.53 | 0.26 |
| BVMT-R | 2.53 ± 7.41 | -1.61 ± 7.83 | 0.16 |
| PASAT | 6.20 ± 16.88 | -4.15 ± 16.33 | 0.11 |
| COWAT | 2.46 ± 9.11 | -0.92 ± 8.99 | 0.33 |
| DKEFS-descriptive | -0.93 ± 2.66 | -1.08 ± 2.18 | 0.79 |
| DKEFS-sorting | -3.46 ± 11.37 | -3.53 ± 9.42 | 0.98 |
| JLO | 3.80 ± 4.10 | 0.23 ± 4.36 | 0.03 |
| CVLT-II-delay | -2.53 ± 11.61 | -0.92 ± 4.19 | 0.74 |
| BVMT-R-delay | 0.93 ± 4.02 | -1.00 ± 2.79 | 0.15 |
Comparing the Effects of Supplementation on Minimal Assessment of Cognitive Function in Multiple Sclerosis Scores Between the Two Study Groups
The results of the within-group analysis are reported in Table 4. The SDMT and JLO scores after intervention in the LEV group were significantly higher than the baseline scores (52.33 ± 10.80 vs. 42.20 ± 15.60, P = 0.01 for SDMT; 21.73 ± 2.31 vs. 17.93 ± 4.21, P < 0.01 for JLO). These results were not observed in the placebo group (P > 0.05).
| Variables | Intervention Group | P-Value a | |
|---|---|---|---|
| LEV | Placebo | ||
| CVLT-II scores | |||
| Before | 46.20 ± 13.41 | 49.46 ± 9.47 | 0.39 |
| After | 50.92 ± 15.24 | 44.61 ± 13.44 | 0.07 |
| SDMT score | |||
| Before | 42.20 ± 15.60 | 52.33 ± 10.80 | 0.01 |
| After | 48.46 ± 17.55 | 52.46 ± 18.12 | 0.34 |
| BVMT-R score | |||
| Before | 17.33 ± 8.06 | 19.86 ± 7.52 | 0.20 |
| After | 23.30 ± 10.14 | 21.69 ± 9.20 | 0.47 |
| PASAT score | |||
| Before | 28.87 ± 22.15 | 35.07 ± 20.37 | 0.27 |
| After | 35.15 ± 20.72 | 31.00 ± 21.30 | 0.64 |
| COWAT score | |||
| Before | 30.86 ± 12.14 | 33.33 ± 9.55 | 0.31 |
| After | 28.46 ± 8.96 | 27.53 ± 12.29 | 0.71 |
| DKEFS-descriptive score | |||
| Before | 6.47 ± 2.26 | 5.53 ± 1.64 | 0.28 |
| After | 6.54 ± 2.03 | 5.50 ± 2.16 | 0.10 |
| DKEFS-sorting score | |||
| Before | 25.07 ± 9.85 | 21.60 ± 6.51 | 0.37 |
| After | 25.23 ± 7.51 | 21.69 ± 10.95 | 0.13 |
| JLO score | |||
| Before | 17.93 ± 4.21 | 21.73 ± 2.31 | < 0.01 |
| After | 20.69 ± 5.29 | 20.92 ± 5.34 | 0.85 |
| CVLT-II-delay score | |||
| Before | 13.13 ± 12.79 | 10.60 ± 2.90 | 0.87 |
| After | 11.15 ± 2.51 | 10.23 ± 3.72 | 0.56 |
| BVMT-R-delay score | |||
| Before | 7.40 ± 4.06 | 8.33 ± 3.01 | 0.38 |
| After | 9.85 ± 3.08 | 8.85 ± 3.00 | 0.12 |
Comparing the Minimal Assessment of Cognitive Function in Multiple Sclerosis Scores Before and After Intervention Among Each Study Group
5. Discussion
A total of 28 patients completed the study. The change in JLO score in the LEV group was significantly greater than in the placebo group. The changes observed in the CVLT-II, BVMT-R, PASAT, COWAT, and BVMT-R-delay subtests in the intervention group increased, while these changes in the placebo group showed a decreasing trend (P > 0.05). Although the differences in these changes were not statistically significant between the two study groups, they are valuable from a clinical perspective. The SDMT and JLO scores after intervention in the LEV group were significantly higher than the baseline scores, while no such improvements were observed in the placebo group. Adverse reactions were minimal, with 10 patients in the placebo group and 12 patients in the LEV group reporting no adverse reactions. The most commonly recorded symptoms in the LEV group were gastrointestinal issues and confusion.
This study evaluated the effects of LEV in improving cognitive disorders and simultaneously assessed seven subgroups of the MACFIMS test. As previously mentioned, there is no known treatment available for cognitive impairment in MS patients.
Clinical studies with donepezil (an acetylcholinesterase inhibitor) in MS patients showed that this drug does not significantly improve cognitive impairment in MS patients (37).
Studies with memantine (NMDA receptor antagonist) and rivastigmine (acetylcholinesterase inhibitor) have not shown any significant difference between the treatment and placebo groups. Moreover, memantine's side effects have been notable (38, 39). Similarly, studies on amantadine, pemoline, and Ginkgo biloba have yielded disappointing results in improving cognitive impairments (40, 41).
Lis-dexamphetamine (LDX) has demonstrated potential in improving thinking skills, particularly processing speed and memory, in people with MS. A phase II study found that patients who took LDX had better cognitive performance, particularly on tests like the SDMT and CVLT-II, compared to those who received a placebo (42).
L-amphetamine has also shown improvement in learning and memory in MS patients. However, in a study where the treatment duration with L-amphetamine was only 14 days, long-term research was challenging due to amphetamine's effects on mood, which complicates the design of extended trials (43, 44).
Morrow et al. examined the effects of fampridine-SR on cognitive fatigue (CF) in MS patients. The results showed that fampridine-SR did not provide significant benefits compared to a placebo in reducing CF. However, the study highlighted the complexity of CF in MS and the need for further research (45).
Numerous studies have investigated the efficacy of disease-modifying drugs (DMDs) in improving cognitive disorders in MS patients (5-12). Unfortunately, drugs such as natalizumab, alemtuzumab, IFNB1-a, IFNB1-b, glatiramer acetate, and fingolimod have primarily helped in maintaining cognitive function rather than improving it. Among these, alemtuzumab has shown some potential in improving information processing, but the effects have been modest and limited (40-42).
Despite numerous studies aiming to find effective treatments for cognitive disorders, no definitive conclusions have been reached in this field. The studies conducted so far often focus on limited aspects of cognitive function or involve short-term evaluations of patients. In 2020, experts recommended conducting high-quality clinical trials to identify effective treatments for cognitive impairment in MS patients (42).
Piracetam and LEV share similar pyrrolidone derivatives and chemical structures. Since piracetam has shown neuroprotective effects in studies and has been somewhat effective in treating cognitive impairments caused by cerebrovascular damage, trauma, and alcohol-related cognitive impairments, it is reasonable to hypothesize that LEV may also positively impact cognitive impairment. Both drugs act as modulators within the CNS, suggesting shared mechanisms of benefit.
As previously mentioned, LEV has demonstrated positive effects on cognitive functions in patients with high-grade glioma, intracranial hemorrhage, and HAPP transgenic mice (which simulate Alzheimer’s disease) (13, 16, 43, 44). Additionally, LEV has shown protective effects against cognitive impairment and white matter damage in cases of long-term brain hypoperfusion in mice (46).
Furthermore, a 2020 study in children with epilepsy found that LEV improved cognitive function, subsequently enhancing the quality of life for epilepsy patients (46). This evidence supports the potential utility of LEV in addressing cognitive impairments in various clinical settings.
It seems that, based on the results obtained from this study and the existing knowledge about LEV, this drug has positive effects on SV2A, modulates the function of pre-synaptic calcium channels, and influences the signaling of GABA and glutamate receptors, impacting certain aspects of cognitive function.
A study published in 2023 demonstrated that LEV improves cognitive impairment caused by streptozotocin in rats. Additionally, in vitro studies revealed that LEV inhibits the polarization of microglia through the JNK/MAPK/NF-KB signaling pathway (47).
Another 2023 animal study showed that LEV might reduce memory loss associated with neuroinflammation by increasing cholinergic activity and reducing neuroinflammation, cell apoptosis, and oxidative stress (48).
In this study, we evaluated the effect of LEV on cognitive impairment in patients with RRMS based on the MACFIMS. The results demonstrated that the SDMT and JLO scores after intervention in the LEV group were significantly higher than the baseline scores. These improvements were not observed in the placebo group. Furthermore, the change in JLO score in the LEV group was significantly greater than that in the placebo group.
5.1. Conclusions
Based on the results of this study and the current knowledge about LEV, the drug appears to have positive effects on SV2A, modulating the function of pre-synaptic calcium channels and the signaling of GABA and glutamate receptors, which are associated with certain cognitive functions. LEV seems effective in some cognitive domains, such as speed of information processing, working memory, and visual-spatial abilities.
The JLO score in the LEV group was significantly greater compared to the placebo group, and both SDMT and JLO scores were significantly higher than baseline scores in the LEV group after the intervention. Despite these promising findings, based on the results and the currently limited data available, it is not possible to recommend LEV as a treatment to improve cognitive impairment in patients with RRMS at this time.
5.2. Limitations
This study had several limitations:
- The follow-up period was relatively short, which may not have been sufficient to fully evaluate the long-term effects of LEV on cognitive impairment.
- While the results indicated an improving trend in the group receiving the drug, these findings were not statistically significant.
- This was a pilot study with only 16 patients in each group, limiting the statistical power of the analysis.
To confirm the findings of this study, further research is required with:
- A longer follow-up period to better assess the drug's effects.
- A larger sample size to enhance the reliability of results.
- Multicenter studies to improve generalizability and validate these findings in diverse settings.
These steps are crucial for determining the true efficacy of LEV in addressing cognitive impairment in RRMS patients.