1. Background
Alveolar macrophages constitute over 90% of the pulmonary macrophage population (1) and have a significant impact on the initiation of inflammatory responses in the lower respiratory tract (2). They secrete an assortment of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6), which are implicated in the pathogenesis of pulmonary disorders such as bacterial pneumonitis (3), chronic obstructive pulmonary disease (COPD) (4, 5), and sarcoidosis (6-11). Lipopolysaccharide (LPS), a critical constituent of the cell wall structure in gram-negative bacteria, induces alveolar macrophages to express TNF-α messenger RNA (mRNA), ultimately leading to bacterial pneumonitis (3). Long-term exposure to cigarette smoke induces alveolar macrophages to overexpress TNF-α, contributing to elevated concentrations of this cytokine in the sputum of patients with COPD (4). The augmented release of TNF-α by cultivated alveolar macrophages obtained from patients with sarcoidosis (6, 7, 10, 11) further highlights the detrimental properties of TNF-α in pulmonary disorders.
Nitric oxide (NO), a free radical generated by various cell types in the lung, also plays an important role in initiating inflammatory responses associated with pulmonary disorders (12-14). Rat alveolar macrophages produce excessive amounts of NO in response to inflammatory stimuli such as LPS. Following exposure of alveolar macrophages to intra-tracheal LPS, up-regulation of inducible nitric oxide synthase (iNOS) occurs, leading to elevated levels of NO (12). Generally, LPS-induced overproduction of TNF-α and NO in various macrophages contributes to the pathogenesis of inflammation.
Isatin, an endogenous compound, exhibits significant anti-inflammatory activity (15). Research by Matheus et al. reveals considerable inhibitory activity of isatin against the production of TNF-α and NO in a murine macrophage cell line (16). The suppression of neuroinflammation mediated by isatin-based compounds against TNF-α and NO in activated macrophages, known as microglia, further emphasizes the potential of isatin derivatives as candidates for treating inflammatory diseases (17).
The LPS-induced activation of alveolar macrophages is characterized by a significant increase in intracellular calcium concentration, an influential factor initiating the overproduction of TNF-α. Wheeler et al. provide pharmacologic evidence that dietary glycine exerts anti-inflammatory effects through modulating activity at glycine-gated channels in alveolar macrophages. The attachment of glycine to these channels leads to a significant decrease in intracellular calcium concentration, subsequently suppressing the release of TNF-α and following inflammatory responses (18, 19).
Given the critical role of TNF-α and NO in the pathogenesis of inflammatory-mediated pulmonary diseases such as bacterial pneumonitis, COPD, and sarcoidosis, developing cytokine suppressive agents is a logical approach to treatment.
2. Objectives
Therefore, a novel series of derivatives 3a-c were designed, synthesized, and biologically evaluated. The reported suppressive activity of isatin and glycine against TNF-α and NO inspired the combination of these compounds to synthesize final derivatives 3a-c (Figure 1). Their inhibitory activity against TNF-α and NO generation in rat alveolar macrophages was assessed and compared with indomethacin as the reference anti-inflammatory drug.
3. Methods
3.1. Chemistry
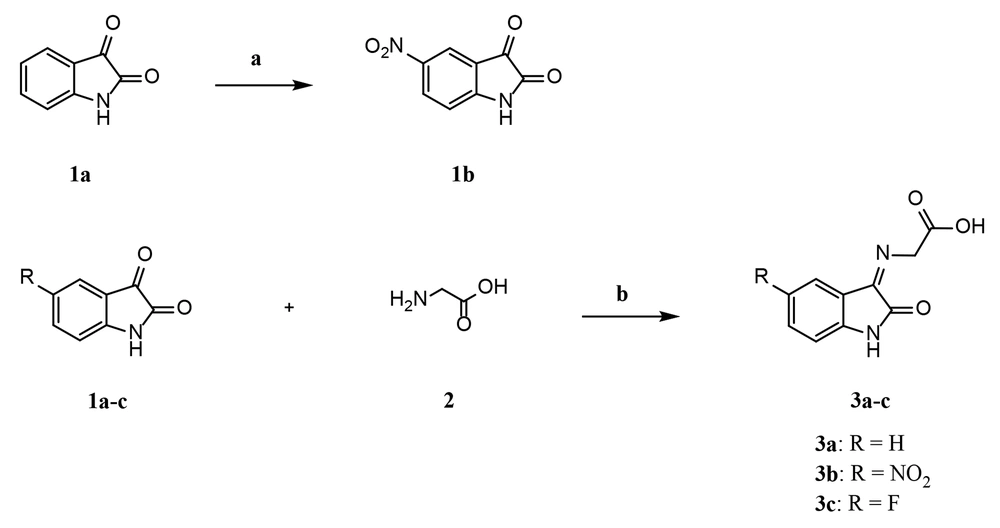
The strategy implemented to prepare final derivatives 3a-c is illustrated in Figure 2. Nitration of isatin 1a at the 5-position was carried out in the presence of potassium nitrate and concentrated sulfuric acid. Imine-based compounds 3a-c were synthesized through the condensation of primary amine 2 with ketones 1a-c. Dry ethanol was used as the solvent, and glacial acetic acid served as the catalyst.
3.2. General Chemistry
All starting materials, reagents, and solvents were obtained from Merck and Aldrich companies without any purification. The reaction progress and the purity of synthesized compounds were monitored by thin-layer chromatography (TLC) on silica gel 250-micron F254 plastic sheets. According to the manufacturer’s instructions, the uncorrected melting points of the final derivatives were determined using a Thermoscientific A9300 apparatus. IR spectra were obtained on a Perkin-Elmer Spectrum using KBr disks. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker 300 (TMS as IS), with chemical shifts reported in parts per million (ppm), downfield from TMS. Proton coupling patterns were presented as singlet (s), multiplet (m), and broad (b). Mass spectra were run on a Finigan TSQ-70 spectrometer (Finigan, USA) at 70 eV. Elemental analysis for C, H, and N was conducted using an Elemental Analyzer GmbH VarioEL.
3.2.1. 5-Nitroindolin-2, 3-dione (1b)
Under ice-bath conditions, potassium nitrate (514 mg, 5.08 mmol) was added portion by portion to a solution of compound 1a (500 mg, 3.39 mmol) and H2SO4 (30 mL) over 15 minutes. The resultant reaction mixture was stirred at ambient temperature for 3 hours. Then, icy cold water was added, and the freshly precipitated yellow solid was filtered and allowed to dry, yielding the desired compound 1b, 300 mg in 45% yield. The yellow solid had a melting point of 252 - 256°C. The IR spectrum (KBr, cm-1) showed peaks at 1336 (N-O symmetric stretching), 1471 (C = C stretching), 1533 (N-O asymmetric stretching), 1771 (C = O stretching), 3095 (C-H stretching), and 3335 (N-H stretching). Elemental analysis calculated for C₈H₄N₂O₄: C, 50.01; H, 2.10; N, 31.55. Found: C, 50.13; H, 2.17; N, 14.52.
3.2.2. General Procedure for Synthesis of Final Derivatives 3a-c
A non-equimolar mixture of glycine (2), the related isatin derivative (1a-c), and glacial acetic acid (1 to 4 drops) in dry ethanol was refluxed under an argon atmosphere for 2 hours, with a molar ratio of 2:1a-c = 4:1. The successful completion of the reaction was confirmed by the TLC technique, and the precipitated glycine was separated through filtration. The filtrate was concentrated under reduced pressure, and the residue was taken up in ethyl acetate and water. The aqueous layer was discarded, and the organic layer was washed with a supersaturated NaCl solution and dried with anhydrous sodium sulfate. After filtration, the ethyl acetate was evaporated in vacuo, and the remaining oily substance was recrystallized from an EtOH/H2O mixture to obtain compounds 3a-c.
3.2.2.1. (Z/E)-2-((2-Oxoindolin-3-Ylidene) Amino) Acetic Acid (3a)
Pink solid, yield: 34%; melting point: 215 - 217°C. IR (KBr, cm-1) ῡ: 1471 (C = C stretching), 1620 (C = C stretching), 1718 (C = O stretching, carboxylic acid), 2800 - 3600 (O - H stretching, carboxylic acid). 1H NMR (300 MHz, DMSO-d6) δ: 10.23 (bs, 1H, OH carboxylic acid), 6.56 - 7.21 (m, aromatic). MS (m/z, %): 204 (M+, 0.34), 112 (20), 58 (37), 45 (41), 42 (100). Anal. Calcd. for C10H8N2O3: C, 58.82; H, 3.95; N, 13.72. Found: C, 58.70; H, 4.13; N, 13.86.
3.2.2.2. (Z/E)-2-((5-Nitro-2-Oxoindolin-3-Ylidene) Amino) Acetic Acid (3b)
Brown solid; yield: 37%; melting point: 247 - 250°C. IR (KBr, cm-1) ῡ: 1340 (N-O symmetric stretching), 1480 (C = C stretching), 1523 (N-O asymmetric stretching), 1625 (C = C stretching), 1734 (C = O stretching, carboxylic acid), 2800-3600 (O-H stretching, carboxylic acid). 1H NMR (300 MHz, DMSO-d6) δ: 10.54 (bs, 1H, OH carboxylic acid), 7.87 - 8.30 (m, aromatic), 6.77 - 7.07 (m, aromatic). MS (m/z, %): 158 (8), 131 (9), 108 (27), 45 (91), 42 (100). Anal. Calcd. for C10H7N3O5: C, 48.20; H, 2.83; N, 16.86. Found: C, 48.08; H, 2.95; N, 16.97.
3.2.2.3. (Z/E)-2-((5-Fluoro-2-Oxoindolin-3-Ylidene) Amino) Acetic Acid (3c)
Brown solid; yield: 28%; melting point: 240 - 243 °C. IR (KBr, cm-1) ῡ: 1486 (C = C stretching), 1629 (C = C stretching), 1722 (C = O stretching, carboxylic acid), 2929 (C-H stretching), 2800 - 3600 (O-H stretching, carboxylic acid). ¹H NMR (300 MHz, DMSO-d6) δ: 10.25 (bs, 1H, OH carboxylic acid), 6.50-7.23 (m, aromatic). MS (m/z, %): 222 (M⁺, 0.38), 97 (22), 81 (9), 69 (51), 45 (23), 43 (100). Anal. Calcd. for C10H7FN2O3: C, 54.06; H, 3.18; N, 12.61. Found: C, 54.22; H, 3.38; N, 12.77.
3.3. Isolation of Alveolar Macrophages
According to the procedure reported by Fereol et al., the separation of alveolar macrophages from healthy male Wistar rats was achieved (20). After anesthesia with pentobarbital sodium (200 mg/kg ip), bronchoalveolar lavage was performed using a lavage solution containing NaCl 140 mM, KCl 5 mM, phosphate buffer solution 2.5 mM, Hepes 10 mM, D-glucose 6 mM, and EDTA 0.2 mM. To collect accurate cellular content, 25 mL of lavage solution was used five times for each rat. Cells were harvested by centrifugation at 500 × g for 10 minutes and re-suspended in a culture medium containing DMEM supplemented with 10% charcoal-stripped FBS and 1% Penicillin/Streptomycin solution. Cells were then counted using the Trypan Blue method and allowed to adhere for 3 hours at 37°C with 5% CO2. After 3 hours of incubation, non-adherent cells were aspirated, and fresh complete medium was added to the attached cells.
3.4. Study Design
The isolated alveolar macrophages, after counting, were seeded in a 96-well plate and grouped as follows: The control group, which received only the culture medium and the vehicle of study compounds; the LPS group, which was exposed only to bacterial-derived LPS (10 μM); the positive control group, which was exposed to LPS and 5 mM indomethacin; and all other test groups, which were exposed to compounds 3a, 3b, and 3c (5 mM) plus LPS.
3.5. The Inhibitory Activity Against Tumor Necrosis Factor-α Production
The isolated alveolar macrophages were seeded in a 96-well plate at a density of 3000 cells per well. After 24 hours, the cells were exposed to bacterial-derived LPS (10 μM) alone or in combination with indomethacin and/or test compounds (5 mg/mL) for an additional 24 hours. To measure the concentrations of TNF-α in the supernatant fluid, an ELISA assay was performed according to the manufacturer's instructions (R&D SYSTEMS, USA).
A 100 μL of capture antibody was added to each well of the 96-well plate and incubated at room temperature overnight. Thereafter, the capture antibody was discarded, and the wells were washed twice with washing buffer (100 μL phosphate buffer saline and 50 μL Tween 20). Following the blocking of wells with diluted reagent (1%, v/v), incubation for 1 hour at room temperature, and washing with washing buffer, 100 μL from each sample or standard was added to each well and incubated at room temperature for 2 hours. After 2 hours of incubation and washing with washing buffer, 100 μL of detection antibody was added to each sample/standard and incubated for a further 2 hours at room temperature.
The assay continued by discarding the detection antibody, washing, and adding 100 μL of streptavidin-HRP solution, followed by 10 minutes of incubation at room temperature. After 10 minutes of incubation and washing with washing buffer, 100 μL of substrate solution was added to each well. After 20 minutes of incubation in a dark place at room temperature, 50 μL of stop solution was added, and the absorbance of each well was read at 450 nm. The concentrations were reported as pg/mL.
3.6. The Inhibitory Activity Against Nitric Oxide Production
As described earlier, isolated alveolar macrophages were exposed to LPS and test compounds. After 24 hours of exposure in triplicates and repeating the assay three times, the measurement of NO levels was performed. The content of NO was accurately measured in the supernatant fluid using the Griess assay (21). The mechanism of the Griess reaction involves the initial conversion of NO into a more stable nitrite (NO2-). When the primary aromatic amine (sulfanilamide) is treated with the nitrite in an acidic solution, a diazonium salt is formed. The reaction of the diazonium salt with a reagent called N-(1-naphthyl) ethylenediamine generates an azo dye compound. As a conjugated molecule, the azo dye compound absorbs electromagnetic radiation in the visible (VIS) region of the spectrum (400 - 750 nm). The exact intensity of absorbance of the conjugated system was recorded with a microplate reader (Bio Tek Epoch 2 Reader) at a test wavelength of 540 nm. According to the mechanism of the Griess assay, the NO content is directly proportional to the intensity of absorbance, meaning a higher NO content corresponds to a higher intensity of absorbance.
3.7. Statistical Analyses
The results were expressed as mean ± standard deviation (M ± SD) from three independently conducted experiments, with triplicates for each group. The obtained data were analyzed using GraphPad Prism (version 7.0; GraphPad Software Inc., San Diego, USA). Comparisons between groups were made using analysis of variance (ANOVA), followed by the Bonferroni post hoc test. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. The Inhibitory Activity Against Tumor Necrosis Factor-α Production
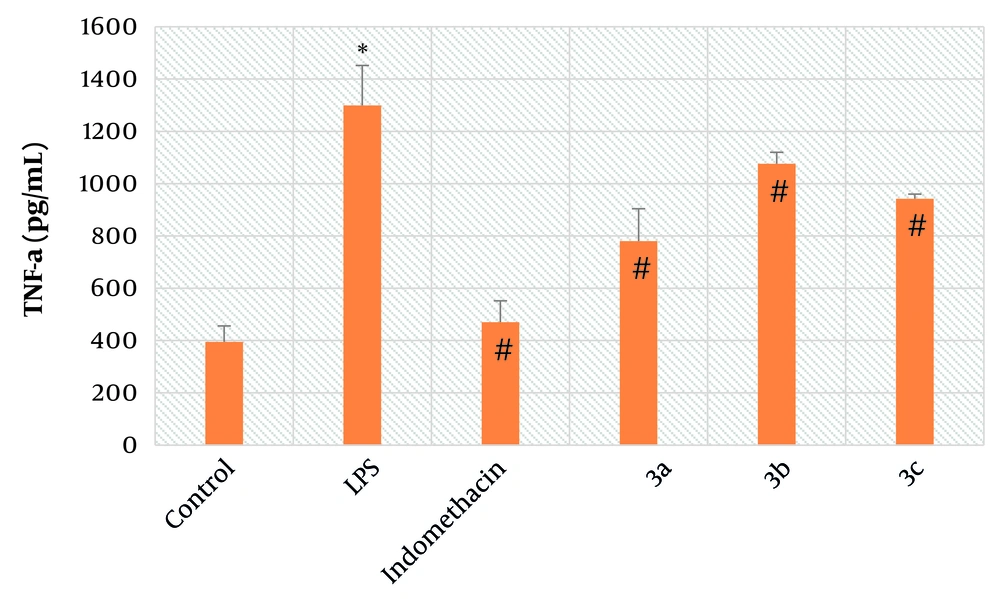
Rat alveolar macrophages were used to assess the inhibitory activity of the final derivatives against LPS-induced overproduction of TNF-α. As shown in Figure 3, exposure of alveolar macrophages to LPS resulted in a significant increase in the concentration of TNF-α compared to the control group (P < 0.01). Another notable finding is the significant inhibitory activity of synthesized compounds 3a-c (P < 0.01) against the overexpression of TNF-α in LPS-exposed alveolar macrophages, which is comparable to indomethacin, the reference anti-inflammatory drug (P < 0.01).
Mean average of tumor necrosis factor-α (TNF-α) concentration in alveolar macrophages; Control: It’s indicative of macrophages which did not receive lipopolysaccharide (LPS), test compounds and indomethacin; LPS: It’s indicative of macrophages which received just LPS; indomethacin: It’s indicative of macrophages which received LPS and indomethacin; 3a: It’s indicative of macrophages which received LPS and test compound 3a; 3b: It’s indicative of macrophages which received LPS and test compound 3b; 3c: It’s indicative of macrophages which received LPS and test compound 3c; symbol * is indicative of significant difference between control and LPS groups (P < 0.01); symbol # indicates the significant difference between LPS and treated groups, including indomethacin, 3a, 3b, and 3c (P < 0.01); bars show the mean ± SD (Triplicates); P < 0.05 was considered as significantly different; LPS concentration: 10 μM, indomethacin concentration: 5 mM, 3a concentration: 5 mM, 3b concentration: 5 mM, 3c concentration: 5 mM.
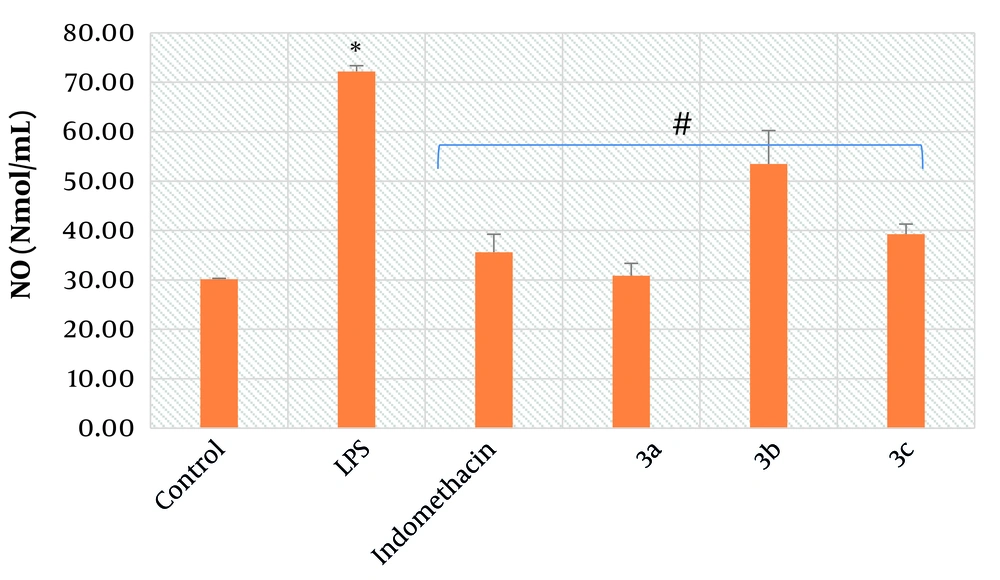
4.2. The Inhibitory Activity Against Nitric Oxide Production
To evaluate the inhibitory activity of the final derivatives against NO production in alveolar macrophages, the Griess assay was implemented. As illustrated in Figure 4, treatment of alveolar macrophages with LPS caused a prominent increase in the concentration of NO compared to the control group (P < 0.01). In line with the findings from Figure 4, a marked decrease in NO levels resulted from the treatment of LPS-exposed macrophages with synthesized compounds 3a-c (P < 0.01) and indomethacin (P < 0.01). Notably, compound 3a exhibited the maximal inhibitory effect. Specifically, after exposure of LPS-exposed alveolar macrophages to 3a, a more significant decrease in the concentration of NO (30.8 Nmol/mL) was observed compared to indomethacin, the reference anti-inflammatory drug, at the same dose (35.5 Nmol/mL).
Mean average of nitric oxide (NO) concentration in alveolar macrophages; control: It’s indicative of macrophages which did not receive lipopolysaccharide (LPS), test compounds and indomethacin; LPS: It’s indicative of macrophages which received just LPS; indomethacin: It’s indicative of macrophages which received LPS and indomethacin; 3a: It’s indicative of macrophages which received LPS and test compound 3a; 3b: It’s indicative of macrophages which received LPS and test compound 3b; 3c: It’s indicative of macrophages which received LPS and test compound 3c; symbol * is indicative of significant difference between control and LPS groups (P < 0.01); symbol # indicates the significant difference between LPS and treated groups, including indomethacin, 3a, 3b, and 3c (P < 0.01); bars show the mean ± SD (Triplicates); P < 0.05 was considered as significantly different; LPS concentration: 10 μM, indomethacin concentration: 5 mM, 3a concentration: 5 mM, 3b concentration: 5 mM, 3c concentration: 5 mM.
5. Discussion
There has been a growing interest in recent years in investigating the anti-inflammatory efficacy of isatin derivatives (22-25). For instance, scientific data reported by Matheus et al. demonstrate that isatin is capable of inhibiting iNOS, which ultimately leads to the disruption of LPS-induced overexpression of NO in murine macrophages. Additionally, data from the corresponding paper highlight the high suppressive activity of isatin against LPS-induced overproduction of TNF-α (16). The meaningful suppression of neuroinflammation mediated by the restrictive activity of isatin-based compounds against TNF-α and NO in activated macrophages, known as microglia, further underscores the potential of isatin derivatives as candidates for treating inflammatory diseases (17).
It is well known that glycine possesses protective activity against hypoxia, ischemia, and various cytotoxic molecules in renal proximal tubules and hepatocytes (26-29). For instance, glycine suppresses LPS-induced overproduction of TNF-α by binding to glycine-gated chloride channels in Kupffer cells, leading to the neutralization of hepatic toxicity (30, 31). In the present study, alveolar macrophages were investigated to evaluate the anti-inflammatory activity of glycine-based derivatives, as their sensitivity to glycine is higher than that of Kupffer cells (IC₅₀ ≈ 10 μM vs. ≈ 300 μM in Kupffer cells) (32).
The LPS-induced activation of alveolar macrophages is typically characterized by a significant increase in intracellular calcium concentration, an influential factor initiating the overproduction of TNF-α (18, 33). The protective influx of extracellular chloride is crucial for opposing the increase in intracellular calcium, ultimately leading to the inactivation of alveolar macrophages (18, 33). Scientific findings by Wheeler and Thurman demonstrate that glycine-induced influx of extracellular chloride neutralizes the calcium increment, suppressing TNF-α release and inactivating alveolar macrophages (18). Since LPS-induced overproduction of TNF-α in alveolar macrophages can trigger a cascade of events leading to chronic lung injury, glycine-based compounds should be considered potential anti-inflammatory agents for patients with pulmonary disorders (19, 33, 34).
Molecular hybridization is a familiar strategy in designing highly efficient drug candidates (35-37). It involves combining pharmacologically active substances to generate a new hybrid compound with an improved pharmacodynamic profile (35-37). As discussed earlier, isatin (16) and glycine (18) can inhibit LPS-induced overexpression of NO and TNF-α in macrophages, respectively. Thus, a rational basis for designing final compounds 3a-c was established. As demonstrated in Figure 1, pharmacologically active molecules, including isatin derivatives and glycine, were combined to generate 3a-c. Theoretically, the novel derivatives 3a-c could simultaneously modulate the biological function of glycine-gated chloride channels and iNOS, leading to the suppression of LPS-stimulated overproduction of TNF-α and NO in alveolar macrophages.
As demonstrated in Figures 3, and 4, treatment of LPS-exposed alveolar macrophages with synthesized hybrid derivatives 3a-c led to a significant decrease in the concentration of TNF-α and NO. These data support the hypothesis that 3a-c may disrupt LPS-induced overproduction of corresponding inflammatory mediators through simultaneous modulation of glycine-gated chloride channels and iNOS. Thus, 3a-c should be regarded as promising lead compounds for treating inflammatory-mediated pulmonary disorders, such as bacterial pneumonitis, COPD, and sarcoidosis. However, to provide more valid results, the synthesis of more derivatives and in vivo studies are required.
5.1. Conclusions
A novel series of hybrid compounds 3a-c were designed, synthesized, and evaluated as potential anti-inflammatory agents. Treatment of LPS-exposed alveolar macrophages with final derivatives 3a-c resulted in a marked decrease in TNF-α and NO levels (P < 0.01), comparable to the effects of indomethacin (P < 0.01). However, due to potential limitations in the present study, such as the limited range of synthesized compounds, we cannot conclusively determine whether 3a-c are valid drug candidates. Nonetheless, they hold promise for further modification as potential lead compounds.