1. Background
When the host encounters foreign challenges or tissue damage, inflammation occurs to restore tissue structure and function (1). Proinflammatory cytokines are produced by immune cells during this process (2, 3). In the innate immune response, macrophages, neutrophils, and fibroblasts play a major role (4). Activated macrophages can produce proinflammatory cytokines (5). One of the substances that can activate macrophages to produce inflammatory mediators such as NO, COX-2, and proinflammatory cytokines is lipopolysaccharide (LPS) (6).
Gnetum montanum is a woody vine 10 to 12 meters long, found across China, India, Thailand, and Vietnam (7). Traditionally, G. montanum is utilized for treating various diseases in Vietnam, such as joint arthritis, gout, and malaria. Gnetum montanum’s stem and rhizomes harbor numerous types of bioactive metabolites such as stilbenes (8), lignans (9), and alkaloids (10), most of which possess anti-inflammatory and antioxidant properties (11, 12). Pinoresinol is a substance found in G. montanum (9). Several studies have shown that pinoresinol has many pharmacological properties, including involvement in hypoperfusion and reperfusion (13), anti-inflammation (14, 15), hepatoprotective effects (16), anti-cancer (17), and anti-fungal properties (18).
2. Objectives
The anti-inflammatory effect of pinoresinol from G. montanum Markgr on macrophages was evaluated in this study. To demonstrate this effect, pinoresinol was extracted from G. montanum Markgr and evaluated for its ability to inhibit pro-inflammatory cytokines, mitogen-activated protein kinase (MAPK) pathways, and reactive oxygen species (ROS).
3. Methods
3.1. Plant Material
Gnetum montanum Markgr specimens were collected from Hoa Binh, Vietnam, and identified (HNU 0245529) by the Museum of Biology, VNU, University of Science.
3.2. Chemicals
Lipopolysaccharide, n-hexane, methanol, ethyl acetate, hydrochloric acid, formic acid, acetonitrile, dichloromethane, dimethyl sulfoxide (DMSO), pinoresinol, and dexamethasone were provided by Merck. Antibodies against extracellular signal-regulated kinases 1 and 2 (ERK1/2), phospho-ERK1/2, p38, and phospho-p38 were provided by Cell Signaling Technology (Beverly, MA). Interleukin (IL)-6 enzyme-linked immunosorbent assay (ELISA) kit, tumor necrosis factor-α (TNF-α) ELISA kit, and IL-1β ELISA kit were provided by BD Pharmingen (Franklin Lakes, NJ).
3.3. Extraction of Pinoresinol from Gnetum montanum Markgr
The extraction method was performed according to the method previously described by Meagher et al. (19). Specifically, the dried powder of G. montanum Markgr (100 g) was extracted with methanol/water (80:20, v/v) (4 g/50 mL) for 4 hours at 55°C. To obtain the residue (8.83 g), the extract was evaporated at 35°C. The residue was then dissolved in water before being hydrolyzed with hydrochloric acid (1 M concentration) for 1 hour at 100°C in a ratio of 10 mL of extract to 0.8 mL of acid. The hydrolysate was then diluted twice with water and further extracted twice with the same volume of ethyl acetate:hexane (1:1). The organic phase was collected, dried, and redissolved with methanol to test for the presence of pinoresinol. The presence of pinoresinol was confirmed by thin-layer chromatography (TLC), using a mobile phase of dichloromethane:acetone:formic acid (80:18:2), and detected by UV at 280 nm (20). The purity of pinoresinol was evaluated by high-performance liquid chromatography (HPLC) using the Shimadzu 20A system (Japan). The mobile phase used two solvent systems: Solvent A (deionized water: 0.1% formic acid) and solvent B (acetonitrile: 0.1% formic acid). The gradient elution process started from 20% to 95% solvent B within 37.5 minutes, then returned to 80%A:20%B within 22.5 minutes. The analyzed sample volume was 20 µL, the flow rate was 1 mL/min, and detection was at 280 nm (20).
3.4. The Culture of Macrophages
Raw 264.7 cells were cultured according to the cell bank instructions (ATCC).
3.5. Assay of Cell Viability
Pinoresinol was dissolved in 10% DMSO. The concentrations of pinoresinol were added to Raw 264.7 cells for 48 hours. Next, 10 μL of CCK-8 was added to each well, followed by 1 hour of incubation. Absorption was measured at a wavelength of 450 nm using a microplate reader (Bio-Rad Inc, USA).
3.6. Enzyme-Linked Immunosorbent Assay
Pinoresinol was incubated with Raw 264.7 cells and then treated with LPS (1 μg/mL) as previously described (21). The determination of cytokines in the supernatant of the cell culture was performed using ELISA kits.
3.7. Western Blotting
Raw 264.7 cells were treated with pinoresinol or 0.1% DMSO, followed by LPS (1 μg/mL). After treatment, the cells were lysed. Primary antibodies used included ERK1/2 (1:1000), phospho-ERK1/2 (1:1000), phospho-p38 (1:1000), and p38 (1:1000). The subsequent steps were performed as previously described (21).
3.8. Assay of Intracellular Reactive Oxygen Species
Reactive oxygen species were quantified as previously described (21). After Raw 264.7 cells were treated with pinoresinol and LPS, 2 µM dihydroethidium (Merck) was added for 15 minutes in 5% CO2 at 37°C. Laser-scanning confocal microscopy (LSM 510) was used to measure ROS.
3.9. Statistical Analyses
The data from three independent experiments are expressed as the mean ± standard deviation (SD). A Student's t-test for independent means was used to determine statistical significance, utilizing Microsoft Excel. Statistical significance was considered valid when P < 0.05.
4. Results
4.1. The Evaluation of Pinoresinol Purity by Thin-Layer Chromatography and High-Performance Liquid Chromatography
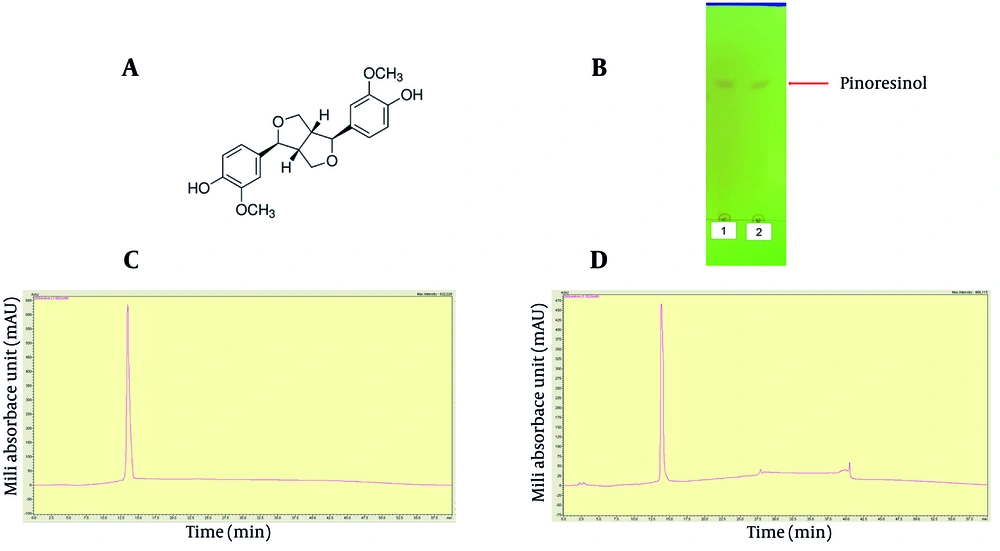
Figure 1A depicts the structure of pinoresinol. Figure 1B shows that the isolated pinoresinol sample from G. montanum Markgr is equivalent to standard pinoresinol as determined by TLC. The purified pinoresinol was compatible with standard pinoresinol in both shape and retention time (Figure 1C, D). The HPLC results using the Shimadzu 20A system indicated that the retention time of pinoresinol extracted from G. montanum Markgr is the same as the standard (13.10 minutes) and consistent with previously published results (20).
Thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) for purified sample and standard pinoresinol; A, chemical structure of pinoresinol; B, 1: TLC for standard pinoresinol; 2: TLC for sample pinoresinol; C, HPLC for standard pinoresinol; D, HPLC for sample pinoresinol.
4.2. The Cytotoxic Effects of Pinoresinol on Cell Viability
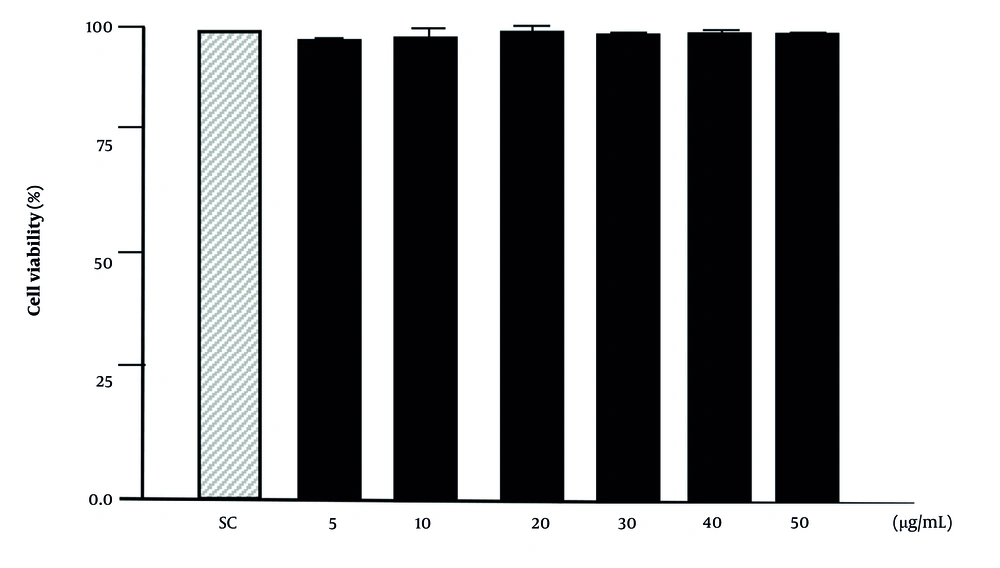
The cytotoxicity of pinoresinol in Raw 264.7 cells was measured using the CCK assay. Pinoresinol at concentrations ranging from 5 µg/mL to 50 µg/mL was not toxic to the cells (Figure 2).
Effect of pinoresinol on the viability of macrophage; the concentrations of pinoresinol were incubated with Raw 264.7 (1 × 106 cells/well). CCK-8 was used to analyze the viability of cells. SC, 0.1% dimethyl sulfoxide (DMSO). Data are presented as mean ± SD of three independent experiments.
4.3. Proinflammatory Cytokine Inhibitory Ability of Pinoresinol
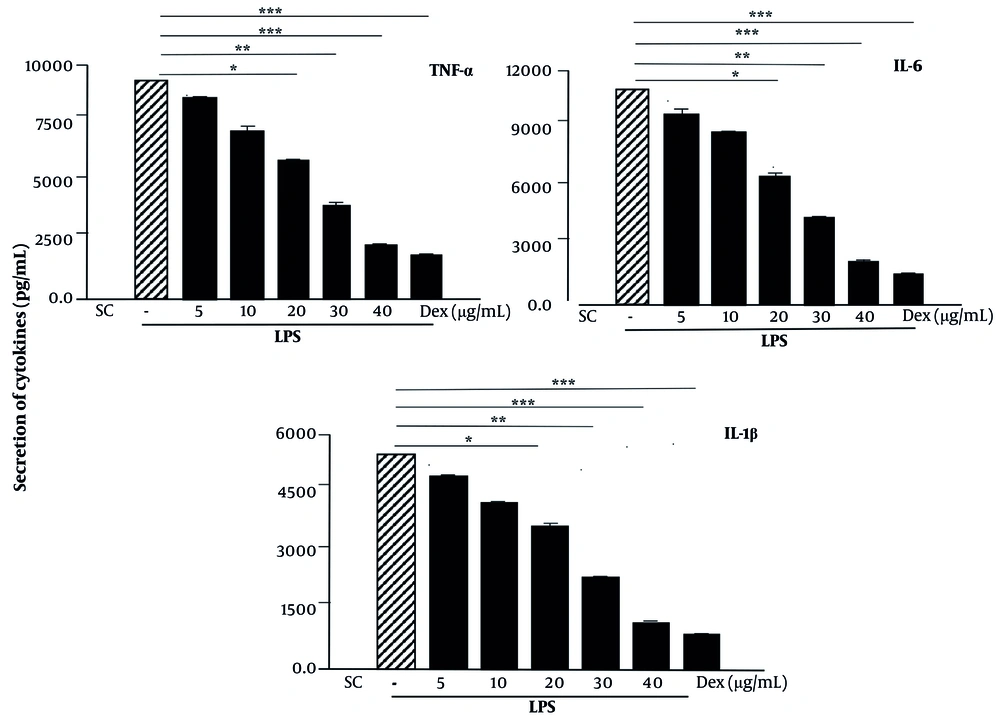
Tumor necrosis factor-α, IL-6, and IL-1β were inhibited by pinoresinol in a concentration-dependent manner. At a concentration of 40 µg/mL (P < 0.001), the release of all the tested cytokines was strongly inhibited in Raw 264.7 cells: TNF-α, IL-6, and IL-1β release decreased by approximately 78.9%, 86.3%, and 81.8%, respectively, compared to the positive control (Figure 3). This inhibition is nearly equal to that of dexamethasone, a potent inhibitor of pro-inflammatory cytokines that is used commercially. Based on this result, 40 µg/mL of pinoresinol will be used for further experiments.
Pinoresinol inhibited the lipopolysaccharide (LPS)-induced proinflammatory cytokines; pinoresinol was treated with Raw 264.7 at different concentrations for 45 min and stimulated with LPS (1 µg/mL) for 18h and supernatants were harvested for enzyme-linked immunosorbent assay (ELISA). Data are presented as mean ± SD of three independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001 compared to the cell group with dimethyl sulfoxide (DMSO). SC, -, 0.1% DMSO; Dex, dexamethasone.
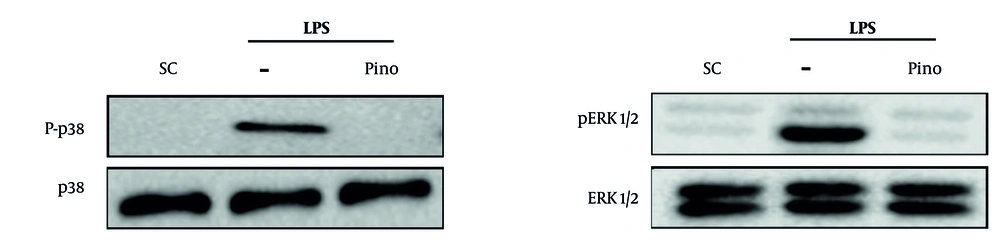
4.4. Pinoresinol Inhibited LPS-Induced Mitogen-Activated Protein Kinase Phosphorylation in Macrophages
The LPS-stimulated MAPK cascade leads to signal transduction in inflammatory models (22). Therefore, the effect of pinoresinol on ERK1/2 and p38 activation was studied. The phosphorylation of p38 and ERK1/2 was decreased in pinoresinol-treated Raw 264.7 cells at 40 µg/mL, as shown in Figure 4 (Appendix 1 in the Supplementary File).
Pinoresinol inhibited the lipopolysaccharide (LPS)-induced mitogen-activated protein kinase (MAPK) activation; Raw 264.7 was incubated with pinoresinol (40 µg/mL) or 0.1% DMSO for 45 min and 30 min of LPS (1 µg/mL) incubation. The MAPK signaling pathways were detected by Western blot. These results are presented from three independent experiments. SC, -, 0.1% dimethyl sulfoxide (DMSO); Pino, pinoresinol.
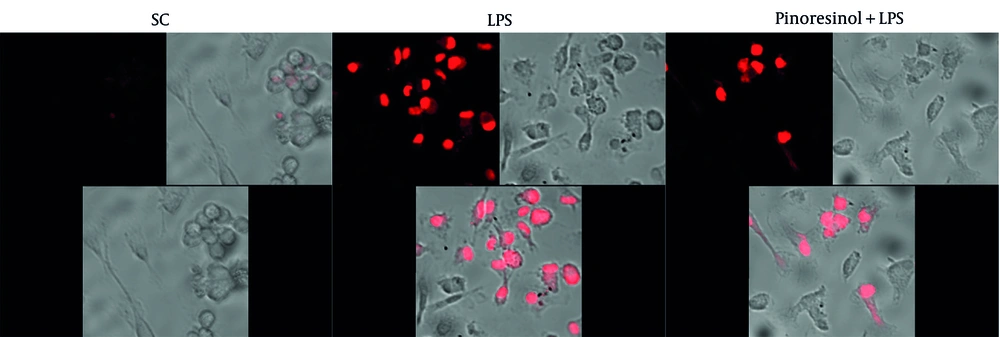
4.5. Pinoresinol Inhibits LPS-Induced Reactive Oxygen Species Secretion
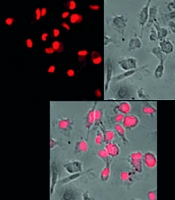
Lipopolysaccharide can stimulate the production of ROS in macrophages (23). Therefore, we investigated the effect of pinoresinol on ROS generation through LPS stimulation in cells. The SC group exhibited a small amount of ROS production, as shown by the weakest color, due to the normal physiological activity of the cells. When the cells were stimulated by LPS, they exhibited strong ROS production (the strongest color). When cells were treated with 40 μg/mL pinoresinol (P < 0.001), the color intensity decreased by approximately 72.4% compared to the LPS-induced inflammation group, indicating that ROS production was significantly inhibited (Figure 5).
Pinoresinol aborted lipopolysaccharide (LPS)-induced reactive oxygen species (ROS) in Raw 264.7; Raw 264.7 was incubated with pinoresinol (40 µg/mL) or 0.1% dimethyl sulfoxide (DMSO) for 45 min and 30 min of LPS (1 µg/mL) incubation. These results are presented from three independent experiments. SC, 0.1% DMSO.
5. Discussion
Pinoresinol belongs to the flavonoid group of lignans in G. montanum. Pinoresinol has been shown to have many important biological activities (13-18). Previous results have demonstrated that pinoresinol inhibits anti-inflammatory activity via NF-κB pathway activation signaling in microglial cells (14, 15). Proinflammatory cytokines are closely related to acute and chronic inflammation (2). Therefore, studying the inhibition of these cytokines is important for controlling inflammatory responses. Here, we demonstrated that proinflammatory cytokines produced in LPS-stimulated macrophages were inhibited by pinoresinol. This result is consistent with previous findings, where pinoresinol also inhibited IL-6 expression in microglia (14).
During the process of protecting the host from infection due to tissue damage, macrophages secrete inflammatory cytokines such as TNF-α and IL-6 (24). However, overproduction of these cytokines leads to severe inflammatory diseases (25). Therefore, inhibiting the production of inflammatory cytokines is considered a treatment strategy for inflammation-related diseases. In both innate and adaptive immune responses, MAPK pathways are involved in inflammatory signaling (26). The LPS-stimulated cytokine production in macrophages involves the IKK-NF-κB and MAPK signaling pathways (22). Several inhibitors of LPS-induced proinflammatory cytokine production have been shown to inhibit NF-κB and MAPK signaling, such as sorafenib, resveratrol, and kaempferol (22, 27, 28).
The p38 MAPK pathway is identified as a major regulator of pro-inflammatory cytokine production in myeloid cells. Many anti-inflammatory drugs target this factor for evaluation in cellular, preclinical, and clinical models of inflammatory disease. The ERK1/2 are the final components of the MAPK phosphorylated layer, an integral module in various signaling pathways that shape cell behavior and fate. The ERK1/2 plays a role in the mechanism of action of immune receptors for inflammatory gene expression in response to infection and cell or tissue damage. In particular, the Toll receptor activates ERK1/2, leading to dysfunction in the inflammatory response and is also a potential target for treating inflammatory diseases.
Indeed, this study demonstrated that pinoresinol inhibited the phosphorylation of p38 and ERK1/2 in LPS-stimulated macrophages. Furthermore, some previous studies have also shown that pinoresinol has the ability to inhibit inflammation through NF-κB (14). These data suggest that the anti-inflammatory potential of pinoresinol is through its ability to inhibit the MAPK pathway and NF-κB activation.
When external pathogens invade the body, ROS are secreted by macrophages to destroy them (29). In a balanced state, ROS do not cause cell damage. However, when this balance is disrupted, excessive ROS can lead to various inflammatory diseases (29). The enhancement of ROS production by leukocytes at the site of inflammation causes endothelial dysfunction and tissue damage. Past and current research in the field of inflammation, with a particular emphasis on oxidative stress-mediated signaling mechanisms associated with inflammation and tissue damage, holds great potential for identifying inflammatory active substances to aid in the treatment of inflammation-related diseases. The ROS play an important role in the MAPK signaling pathways (30). However, the effect of pinoresinol on ROS during inflammation has not been previously published.
Our study demonstrated that pinoresinol-mediated inhibition of ROS activation was LPS-dependent. Together with previous studies, our data further support the potential anti-inflammatory ability of pinoresinol to control ROS. The effective activity of pinoresinol in several diseases, including cancer (17), inflammation (14), and hepatoprotection, has been reported in several recent studies (16). This study further elucidated the anti-inflammatory role of pinoresinol in macrophages in an LPS-dependent manner. When ROS exceed the equilibrium state, they can lead to a number of inflammatory diseases (30). Therefore, bioactive substances with the ability to inhibit ROS can be considered promising anti-inflammatory agents. Recent studies have focused on developing drugs from biological substances that can disrupt the generation of ROS for the treatment of ROS and inflammation in clinical conditions (29). The LPS is used as a model of inflammatory response in the immune system (22). Therefore, the data in this study contribute to the understanding of the inhibitory ability of pinoresinol on inflammatory processes through MAPK and ROS signaling.
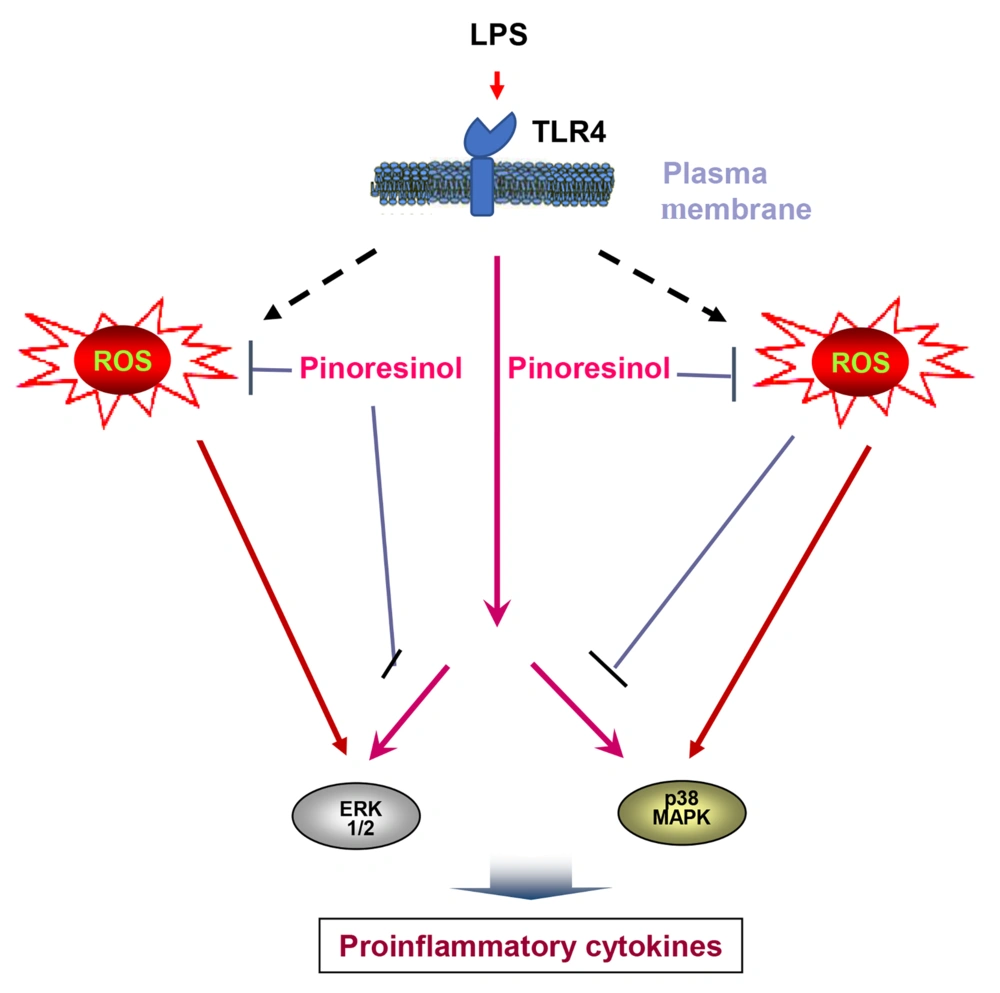
5.1. Conclusions
Lipopolysaccharide induces MAPK and ROS through toll-like receptor (TLR)4. Pre-treatment with pinoresinol effectively abrogates the phosphorylation of p38, ERK1/2, and ROS (Figure 6). This study demonstrated that pinoresinol, isolated from Vietnamese G. montanum Markgr, modulates acute inflammatory status through ROS-dependent pathways. In summary, pinoresinol is a potential agent for the control of inflammatory diseases.