1. Background
Psychological stress has been proven to raise oxidative stress levels, resulting in a range of health issues, including inflammation, alterations in the gut microbiota, metabolic diseases, and cancer (1). Oxidative stress is a state of imbalance between the production of reactive oxygen species (ROS) and the body’s ability to detoxify or repair the resulting damage (2). Psychological stress can increase ROS production through various mechanisms, including activation of the hypothalamic-pituitary-adrenal (HPA) axis, increased sympathetic nervous system activity, and dysregulation of inflammation (3). The gut microbiota supports intestinal health and reduces oxidative stress by producing beneficial metabolites called postbiotics. These postbiotics interact with epithelial and immune cells, exerting effects such as anti-inflammatory, antioxidant, and anticancer activities. However, stress can disrupt the gut microbiota balance, increasing oxidative stress and compromising these protective functions (4). Psychological stress has been shown to induce intestinal dysbiosis by altering the composition and function of the intestinal microbiota (5). Dysbiosis, an imbalance in the gut microbiota, is associated with greater vulnerability to infections and increased systemic inflammation, which in turn contributes to oxidative stress. This relationship is complex and bidirectional, with dysbiosis, inflammation, and oxidative stress influencing each other (6).
Escherichia coli, Salmonella, and Shigella are gram-negative bacteria from the phylum Proteobacteria, with E. coli typically being a normal resident, while Salmonella and Shigella include pathogenic species causing foodborne illnesses and dysentery. Enterococcus, a gram-positive bacterium from the phylum Firmicutes, is normally present in the human gut but can cause serious infections such as urinary tract infections (UTIs), sepsis, and endocarditis (7). Psychological stress can alter gut bacterial populations, as stress hormones like catecholamines promote microbial growth. Norepinephrine, in particular, significantly enhances the growth of commensal E. coli, indicating a direct link between stress and E. coli overgrowth (8). Although no study has examined the effect of chronic psychological stress on Enterococcus populations, in vitro studies using gut model bioreactors show that exposure to certain stressors can boost these bacteria’s presence (9).
Fecal microbiota transplantation (FMT) is a promising therapeutic approach that involves transferring fecal microbiota from a healthy donor to a recipient to restore the gut microbiota balance (10). The FMT has emerged as a potential therapy for various gastrointestinal disorders, including inflammatory bowel disease and recurrent Clostridioides difficile infection (11). The mechanism by which FMT exerts its therapeutic effects is not fully understood, but it is believed that FMT restores the balance of the gut microbiota, leading to improved gut function and reduced inflammation. However, the effect of FMT on oxidative stress and intestinal microbiota following heavy psychological stress has not been investigated (12).
2. Objectives
The present study aims to investigate the effect of FMT on oxidative stress and some opportunistic intestinal microbiota following unpredictable chronic psychological stress in mice.
3. Methods
The present study was conducted using an experimental and case-control method. Thirty-two male Balb/c mice were randomly divided into four groups (n = 8): (1) INTACT (negative control): Did not receive any intervention; (2) control: Received only electric foot shock stress; (3) SHAM: After stress, received normal saline solvent via gavage instead of FMT; (4) FMT-treated group (Tf): After stress, received FMT via gavage.
3.1. Experiment Stages
(1) Electric foot shock stress for 10 consecutive days.
(2) Depletion of gut microbiota for 3 days using antibiotics.
(3) FMT for 8 weeks.
(4) Sacrifice of mice for blood collection to perform the ferric-reducing antioxidant power (FRAP) test and feces collection for microbiota analysis [PurPlate, spread culture, and quantitative real-time PCR (qPCR)].
3.2. Electric Foot Shock Stress
As previously described, mice were placed inside a Plexiglas chamber measuring 26 × 21 × 26 cm (L × W × H) with a grid floor made of stainless-steel rods (3 mm in diameter, spaced 10 mm apart) for a consecutive period of 10 days. They were subjected to a specific paradigm of 30 random 1-second shocks with a strength of 0.4 mA delivered over a 15-minute duration (13).
3.3. Antibiotic Treatment
Mice received penicillin (2000 U/mL) and streptomycin (2 mg/mL) in drinking water for 3 days to eliminate indigenous gut microorganisms. After treatment, 200 mg of fresh feces from the intact group was resuspended in 5 mL of normal saline, vortexed for 3 minutes, and settled by gravity for 2 minutes. Transplantation to recipient mice was performed by gavage with 200 µL of supernatant from a stool sample once a day for 8 weeks.
3.4. Antioxidant Measurement of Total Serum
The FRAP technique was used to measure the level of antioxidants in blood serum, based on reviving antioxidant components and changing their color, followed by measuring the concentration of serum antioxidants using spectroscopic techniques. A ready-purchased 46-piece kit from ZellBio GmbH (Germany) was used (2).
Each mouse was placed in a sterilized, labeled cage for up to 5 minutes to collect 4 - 8 fresh fecal pellets or at least 35 mg of feces, which were then transferred to labeled Eppendorf tubes using a fresh needle. The samples were immediately placed on dry ice and stored at -80°C for further processing. To extract genomic DNA from the collected fecal samples, which took approximately 5 - 7 hours for 32 samples, the following steps were performed: In a biosafety cabinet, 25 mg of the mouse fecal sample was taken, and genomic DNA was prepared using the DNA kit according to the manufacturer’s instructions (QIAGEN). For the control group, the microbial community standard was thawed and vortexed to ensure even mixing. Thirty microliters of microbial standard or 50 µL of nuclease-free water (for the intact group) were added to tubes containing the kit buffer. Bead beating at 5,000 rpm for 30 seconds was used to lyse the samples, followed by DNA extraction per the manufacturer’s instructions and elution with 50 µL of nuclease-free water. DNA purity was evaluated using A260/230 and A260/280 ratios measured with a Nanodrop.
3.5. Examination of Microflora
To assess the E. coli and Salmonella intestinal microflora, mice were dissected, and 2 grams of intestinal contents were dissolved in 10 mL of phosphate buffer. MRS, TSC, and YGC media were used with the spread culture and PurPlate methods, followed by colony counting. For precise testing of specific species and quantification of intestinal Enterococcus faecalis and Shigella microflora using qPCR, a mixture was prepared using 1 µL of forward and reverse primers supplied by Sinacolon Co. in Tehran, Iran. Additionally, 10 µL of 2X RealQ Plus master mix Green from Amplicon Bio in Seoul, South Korea, and 1 µL of extracted DNA were combined. The qPCR reaction was conducted using the Rotor-Gene Q real-time PCR system from QIAGEN GmbH in Hilden, Germany. Transcript expression levels were normalized by comparing them to the β-actin gene. The qPCR was performed with the following components: 12 µL of 2 × SYBR Premix Ex, 1 µL of forward primer, 1 µL of reverse primer (Table 1), 1 µL of Rox Reference Dye, and 1 µL of DNA template, in a total volume of 25 µL.
| Bacteria | Sequence (5’ to 3’) | Temperature °C |
|---|---|---|
| Shigella sonnei | F: AATGCCGTAAGGAATGCAAG CTT | 58 |
| R: GAAGGAGATTCGCTGCT | ||
| Enterococcus faecalis | F: TCAAGTACAGTTAGTCTT | 56 |
| R: GCCATGCGGCATAAACTG |
List of Bacteria Species Primers and Annealing Temperatures. Sequences of Forward (F) and Reverse (R) Primers Used for Quantitative Real-time PCR Analysis are Indicated Specifically
Thermal cycling was performed using the real-time PCR System with the following conditions: 95°C for 5 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 31 seconds. The intensity of the fluorescent dye in the reaction was read automatically during PCR cycling. The measured fluorescent signals were normalized against the ROX reference dye (normalized signal, Rn+) and were used to calculate the net fluorescence increments (ΔRn), where ΔRn = Rn+ - baseline Rn+ (Rn+ during cycles 3 to 12). The cycle threshold (Ct) was defined as the cycle number at which a sample’s ΔRn fluorescence crossed the threshold. The Ct was used to quantify the number of bacteria compared against the standard curve.
The combination of these methods offers several specific advantages for the research: The PurPlate method measures the number of colony-forming units (CFUs), providing an estimate of the number of living bacteria, while qPCR measures the quantity of bacterial DNA, indicating the total number of bacteria, including both viable and non-viable cells. Combining both methods helps in assessing the effectiveness of antibacterial treatments. For example, a treatment might reduce the number of viable bacteria (observed through PurPlate) but not eliminate bacterial DNA (detected by qPCR), indicating residual non-viable cells.
3.6. Data Analysis
Data were classified using Excel 2019 and analyzed with Prism software (version 9) using one-way ANOVA and Tukey’s post-test. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (IR.PNU.REC.1402.198).
4. Results
4.1. Total Serum Antioxidants
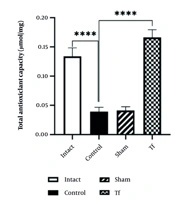
The total serum antioxidants in the control group were significantly lower than in the intact group. The total antioxidant capacity (TAC) in the stressed group (control) was lower than in the intact group (P < 0.0001). The TAC level in the Tf was significantly increased compared to the control group (P < 0.0001) (Figure 1).
The Tf exhibits a significantly higher TAC value than the control group (P < 0.0001), suggesting that FMT positively affects antioxidant capacity.
4.2. Spread Culture and PurPlate Results
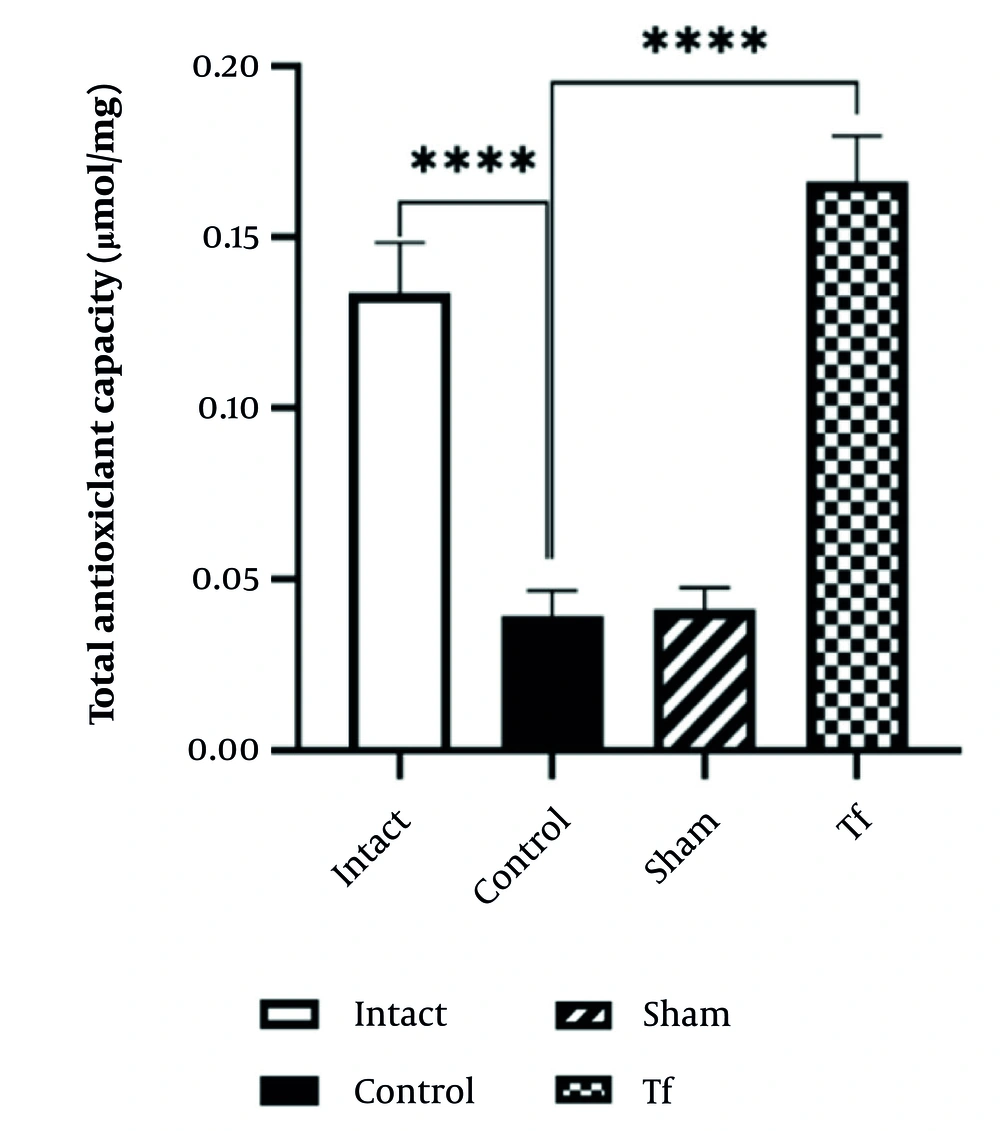
The number of E. coli bacteria increased significantly in the stressed group compared to the intact group (P < 0.0001). However, the number of E. coli bacteria in the FMT-treated group was significantly decreased compared to the stressed group (P < 0.01) (Figure 2).
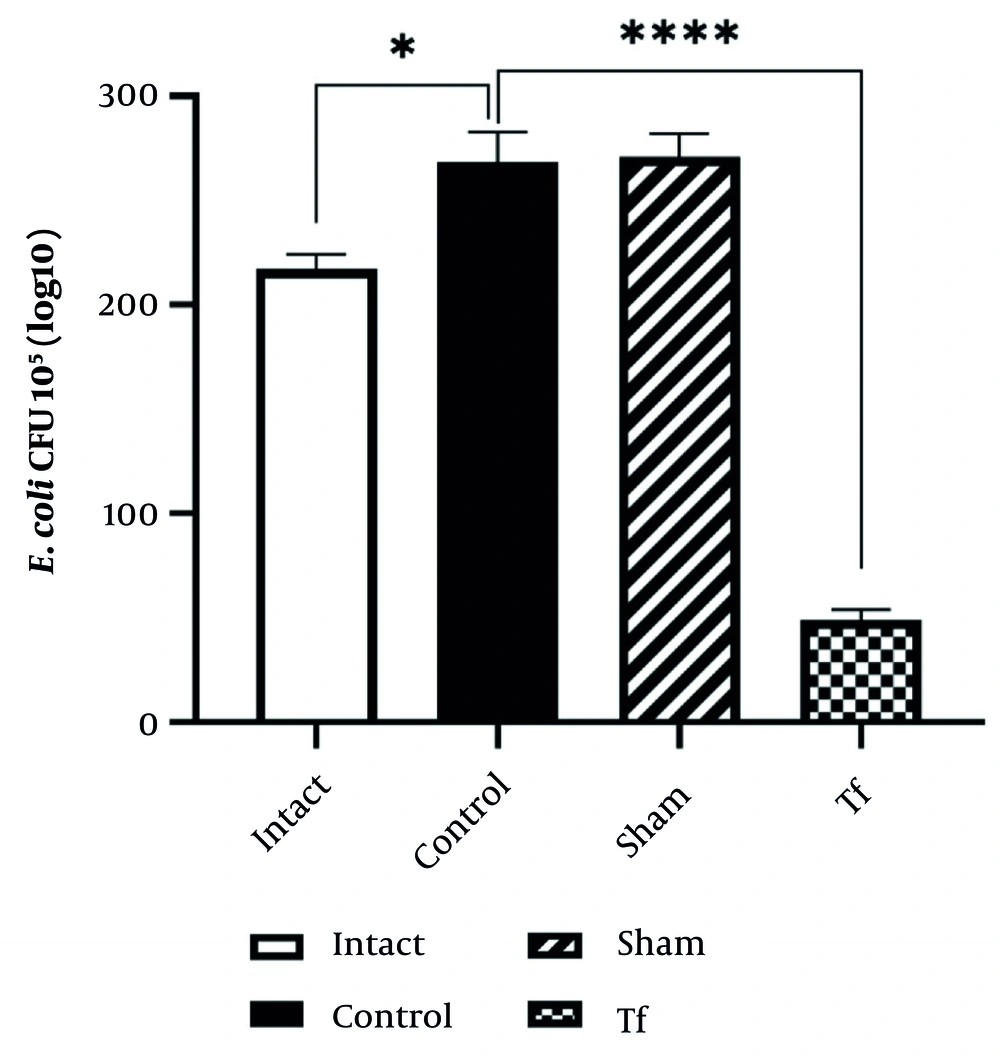
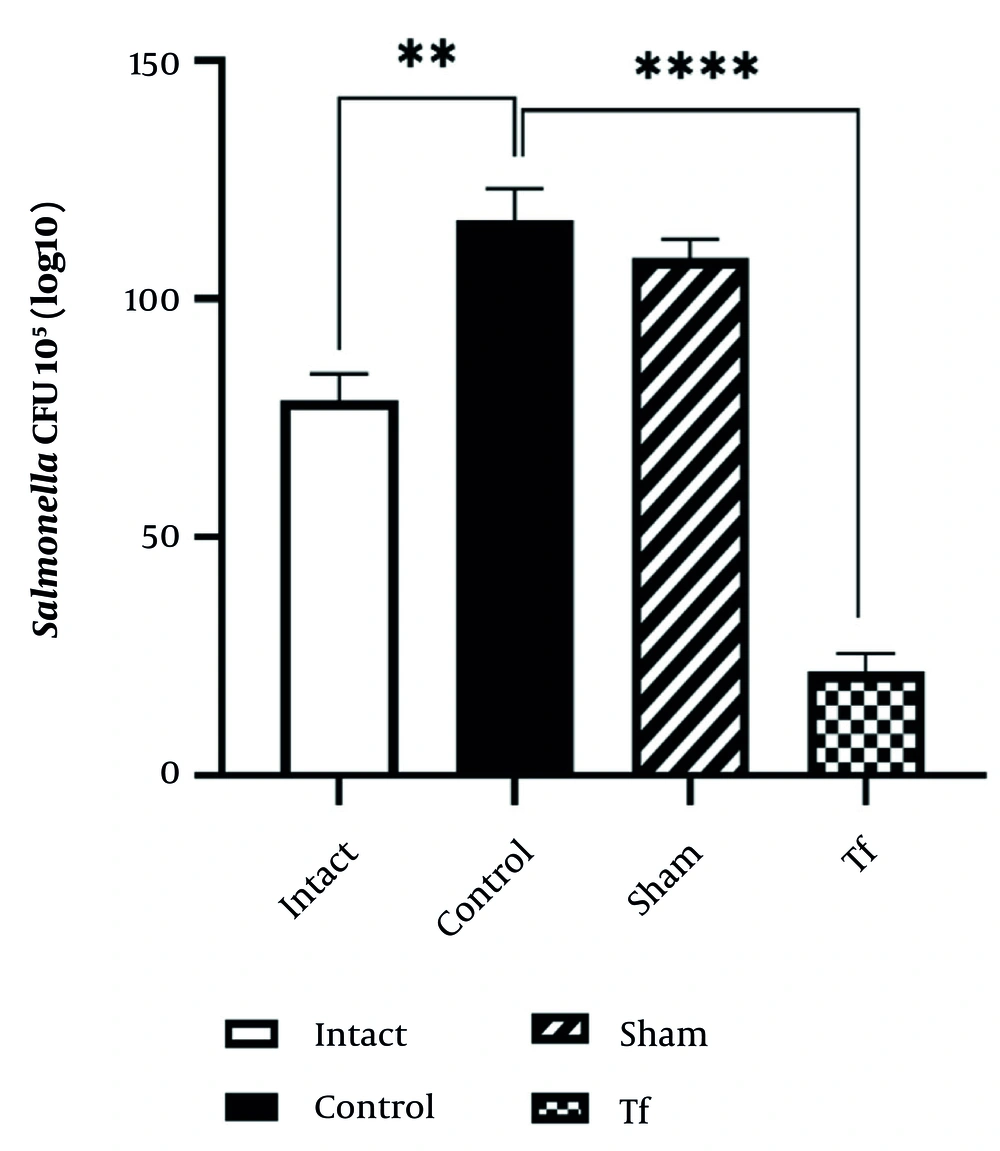
Escherichia coli bacteria significantly increased in the control group compared to the intact group (P < 0.0001). However, E. coli levels significantly decreased in the Tf compared to the control group (P < 0.01). The amount of Salmonella microflora in the control group and intact group, when compared with each other and with the FMT group, showed a significant difference (P < 0.0001) (Figure 3).
The Salmonella CFU count significantly increased in the control group compared to the intact group (P < 0.01). The Tf showed a significant reduction in Salmonella CFU count compared to both the control and sham groups (P < 0.0001), indicating the effectiveness of the Tf treatment in reducing Salmonella levels.
4.3. Quantitative Real-time PCR Results
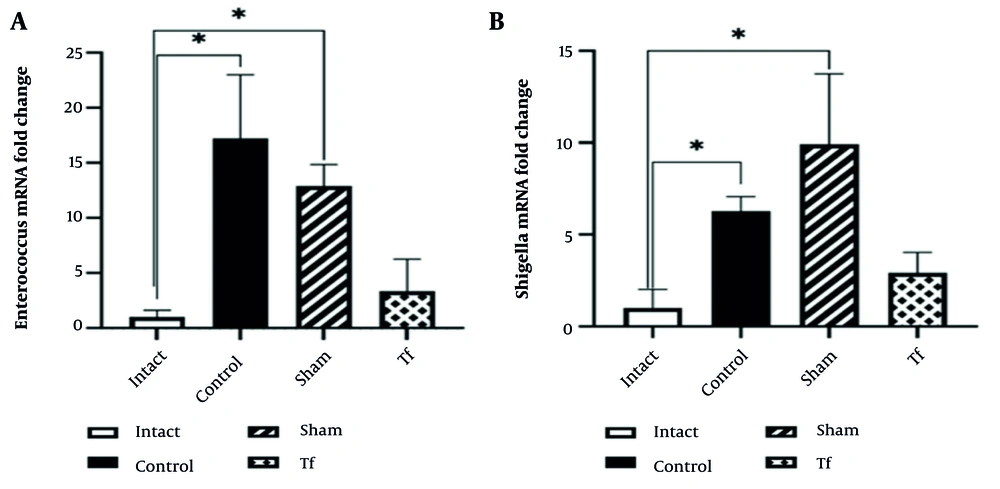
The diagram of Enterococcus mRNA changes in different experimental groups showed that heavy stress on the control group caused a significant increase in Enterococcus mRNA compared to the intact group. The difference between the sham group and the intact group was also significant. Although the difference between these groups and the FMT-receiving group was not significant, the amount of RNA change in the FMT group was not statistically different from the intact group, indicating that FMT was able to reduce the population of Enterococcus to a level similar to the intact group (Figure 4A). This situation was consistent for Shigella mRNA (Figure 4B). The results indicated that FMT recovered the stress-induced increase in the Enterococcus and Shigella populations. The sham group showed a significant difference from the intact group. However, the RNA change in the FMT group was not significantly different from the intact group, indicating FMT reduced Enterococcus to levels similar to the intact group (P < 0.05) (Figure 4A). This was also true for Shigella mRNA (P < 0.05) (Figure 4B). These results suggest that FMT counteracted the stress-induced increase in Enterococcus and Shigella populations.
5. Discussion
This study utilized a mouse model subjected to heavy stress for 10 days to examine the effects of FMT on oxidative stress and gut microbiota. Mice received either FMT or a sham treatment, and TAC in blood serum was assessed using the FRAP technique. Gut microbiota composition was analyzed through 16S rRNA gene sequencing. The findings of this study provide valuable insights into the potential benefits of FMT in mitigating the negative effects of heavy stress on gut health and may have implications for therapeutic applications in managing stress-related disorders. The study offers novel insights into how FMT can potentially reduce oxidative stress induced by psychological stress, specifically electric foot shock stress in mice. This connection between FMT and oxidative stress modulation under such conditions has not been extensively explored before.
Psychological stress can increase the growth of intestinal pathogenic bacteria and decrease the levels of probiotic bacteria through various mechanisms. It also alters the composition of the gut microbiota, creating an environment that favors the growth of pathogenic bacteria like Salmonella and Shigella while inhibiting the growth of probiotic bacteria like Lactobacillus. It dysregulates the immune response, leading to inflammation and oxidative stress in the gut, promoting the growth of pathogenic bacteria and reducing competition from probiotic bacteria (14). Moreover, psychological stress reduces the adhesion of beneficial bacterial species like Lactobacillus, making them more susceptible to elimination. These changes in gut microbiota composition contribute to various health issues associated with imbalances, such as inflammatory bowel diseases, obesity, and metabolic disorders (15).
Psychological stress can alter the integrity of the intestinal barrier, leading to increased gut permeability (leaky gut). This increased permeability allows harmful substances, such as bacterial toxins, to enter the bloodstream, triggering an inflammatory response that can disrupt the balance of the gut microbiota (5). Stress can disrupt intestinal motility, affecting the movement of food and waste through the digestive tract. These changes can lead to altered growth and distribution of gut microbiota, resulting in dysbiosis. Additionally, stress impacts gut neurotransmitter levels like serotonin and GABA, further influencing motility and microbial balance (14).
Psychological stress increases oxidative stress by activating the HPA axis, leading to the release of stress hormones like cortisol, which stimulate the production of ROS and reduce the activity of antioxidant enzymes (16). Dysregulated inflammation during stress results in the production of pro-inflammatory cytokines that increase oxidative stress. Elevated cortisol levels can impact gut microbiota composition by promoting the growth of pathogenic bacteria while reducing beneficial bacteria, thus contributing to dysbiosis (17).
Certain bacterial species, such as Lactobacillus and Bifidobacterium, are responsible for producing antioxidants. The gut microbiota regulates inflammation by producing anti-inflammatory molecules like short-chain fatty acids (SCFAs) and butyrate. One probable mechanism through which SCFAs regulate inflammation is by activating specific receptors on immune cells called G protein-coupled receptors (GPCRs), particularly GPR43 and GPR41. When SCFAs bind to these receptors on immune cells, they can initiate signaling pathways that lead to the production of anti-inflammatory cytokines and the inhibition of pro-inflammatory cytokines. This helps to reduce overall inflammation in the body. Additionally, SCFAs can also interact with epigenetic mechanisms, such as histone deacetylase (HDAC) inhibition. Histone deacetylase inhibition can lead to changes in gene expression in immune cells, promoting an anti-inflammatory state and reducing inflammation. Furthermore, SCFAs have been shown to promote the generation of regulatory T-cells (Tregs), which are essential in maintaining immune homeostasis and preventing excessive inflammation. The Tregs produce anti-inflammatory cytokines and suppress the activity of pro-inflammatory immune cells (18).
Furthermore, certain bacterial species, including Lactobacillus acidophilus, can modulate oxidative stress by increasing the activity of antioxidant enzymes (19). The interaction between the gut microbiota and the host immune system also influences oxidative stress levels (20).
5.1. Conclusions
The FMT has the potential to alleviate the negative effects of intense psychological stress on antioxidant capacity and gut microbiota. This study provides evidence for FMT’s therapeutic potential in managing stress-related disorders by restoring gut microbiota balance and reducing oxidative stress. Further research is warranted to explore the clinical applications of FMT and its mechanisms of action.