1. Background
Ischemia-reperfusion (I/R) injury presents a significant clinical challenge, particularly in the context of acute kidney injury, which is characterized by a rapid decline in renal function. This condition occurs when blood flow to the kidneys is temporarily interrupted (ischemia) and subsequently restored (reperfusion), triggering a series of pathological events that can lead to renal cell dysfunction and death (1). The mechanisms underlying I/R injury are multifactorial, involving oxidative stress, inflammation, and cellular apoptosis. Recent reports indicate that the Wnt/β-catenin pathway plays a critical role in I/R damage, interconnected with nuclear factor-kappa B (NF-κB), hypoxia-inducible factor-1 alpha (HIF-1α) signaling, notch, phosphoinositide-3-kinase (PI3K)/protein kinase B (Akt), and transforming growth factor-β (TGF-β). Additionally, the regulation of neurogenesis and fibrosis is orchestrated by a cross-link between Wnt/β-catenin and N-methyl-D-aspartate (NMDA)/hepatocyte growth factor/mesenchymal-epithelial transition factor (HGF/c-Met) and other inflammatory mediators, thereby regulating inflammation and oxidative stress responses (2).
During the ischemic phase, the absence of oxygen and vital nutrients leads to energy depletion in renal tubular cells, severely impairing their functionality. When blood flow is restored, this paradoxically exacerbates cellular damage through the production of reactive oxygen species (ROS) and the activation of inflammatory pathways (2). Studies have shown that these inflammatory cytokines and chemokines not only exacerbate tissue damage but also disrupt renal microcirculation, perpetuating a cycle of injury (3-5). This dual-phase process significantly contributes to the morbidity and mortality associated with acute kidney injury, highlighting the urgent need for research aimed at developing effective therapeutic interventions.
Research has underscored the potential of natural compounds, particularly antioxidants, in offering protective effects against I/R injury (6). One notable compound is astaxanthin (AST), a super antioxidant carotenoid pigment found in various marine organisms, including microalgae and shellfish. The AST is celebrated for its remarkable antioxidant properties, which are significantly more potent than those of other well-known antioxidants such as vitamin E and vitamin C. Beyond its antioxidant effects, AST also exhibits anti-inflammatory properties (7, 8), positioning it as a promising candidate for the prevention and treatment of I/R-induced renal injury. Previous studies have demonstrated the protective effects of AST solid lipid nanoparticles against kidney I/R (9). This is the first research article to highlight the therapeutic role of naïve AST against renal I/R in rats.
2. Objectives
The current study aims to evaluate the protective effects of AST against renal I/R injury dysregulations, renal function, and histopathology using a rat model.
3. Methods
3.1. Animals
This study involved 30 male rats sourced from the animal facility at Kermanshah University of Medical Sciences. The experimental procedures adhered to the ethical guidelines for animal use and care as outlined by Kermanshah University of Medical Sciences (IR.KUMS.REC.1400.314). The rats were maintained under controlled conditions, with a 12-hour light/dark cycle and a temperature range of 25 - 27°C. They were provided with ad libitum access to water and standard food. The animals were randomly assigned to one of five groups:
3.1.1. Sham Group
This group underwent surgery without inducing ischemia and received intraperitoneal (i.p.) treatment with a drug solvent (5% DMSO) for 7 days.
3.1.2. Ischemia-Reperfusion Group
This group underwent surgery followed by 30 minutes of ischemia, after which they received i.p. treatment with the drug solvent for 7 days.
3.1.3. Astaxanthin Treatment Groups
The third, fourth, and fifth groups underwent surgery and 30 minutes of ischemia, followed by i.p. treatment with AST at doses of 5, 10, and 15 mg/kg, respectively, for 7 days.
3.2. Ischemia-Reperfusion Induced Renal Injury
Following the induction of anesthesia using a ketamine and xylazine mixture (80 mg/kg and 20 mg/kg, respectively), both the right and left kidneys were surgically exposed. Ischemia was induced by applying clamps to the renal artery and vein for 30 minutes. Upon completion of this period, the clamps were carefully removed to initiate reperfusion, and the abdominal incision was subsequently sutured (10). Sham rats underwent similar surgical procedures but without occlusion of the renal artery. For 7 days, the animals received either 5% DMSO or varying doses of AST. After one week, the animals were deeply anesthetized with the ketamine and xylazine mixture (100 mg/kg and 20 mg/kg, respectively). Blood samples were collected directly from the hearts of the animals to assess levels of urea, creatinine, glutathione (GSH), catalase, and nitrite. Additionally, kidney tissue was removed for histological analysis.
3.3. Measurement of Renal Functional Parameters
The renal functional parameters, creatinine and urea levels, were estimated using an auto-analyzer, the RA 1000 from Technicon Instruments, USA (11).
3.4. Measurement of Serum Glutathione/Catalase Levels
To assess the level of GSH in serum, the Ellman method was utilized. In this method, 60 microliters of rat serum were mixed with 100 microliters of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), and 50 microliters of phosphate buffer were added. This mixture was incubated for 10 minutes at 37°C, and then the optical absorbance was measured at a wavelength of 412 nanometers (12).
To evaluate serum catalase levels, the Aebi method was employed, which involves assessing changes in hydrogen peroxide (H2O2) concentration. In this assay, 20 microliters of serum sample were mixed with 100 microliters of 65 mM H2O2 and incubated for 4 minutes. After incubation, the reaction was stopped using ammonium molybdate, and the remaining H2O2 concentration was measured at a wavelength of 405 nanometers (13).
Using the formula: [(Csample - Csham)/Csample] × 100, the percentage difference in catalase and GSH concentrations between the sham group and the sample groups (I/R or AST groups) was calculated.
3.5. Measurement of Nitrite
The level of serum nitrite in serum samples was measured using the Griess method. For this purpose, 100 microliters of serum were mixed with 50 microliters of sulfanilamide solution and incubated in the dark for 5 minutes. Then, 50 microliters of N-(1-naphthyl) ethylenediamine dihydrochloride (NEDD) were added to the mixture and incubated for an additional 30 minutes. After this step, the optical density was measured at a wavelength of 540 nanometers to determine the nitrite concentration, which serves as an indicator of nitric oxide levels (14).
3.6. Histological Analysis
Kidney tissues were fixed in 10% formalin. Following the fixation process, the tissues were embedded in paraffin wax to facilitate sectioning. Serial sections, each approximately 4 - 5 micrometers thick, were carefully cut from the paraffin blocks. These sections were subsequently stained using the Hematoxylin and Eosin (H&E) technique and examined under a light microscope to identify pathological changes (15).
3.7. Data Analysis
The analysis of the results was conducted using Prism software, version 8.0. A one-way ANOVA was performed to assess differences between groups, followed by Tukey’s post hoc tests. The data are reported as mean ± standard error of the mean (SEM), and a significance level of P < 0.05 was considered.
4. Results
4.1. Plasma Creatinine and Urea Concentration
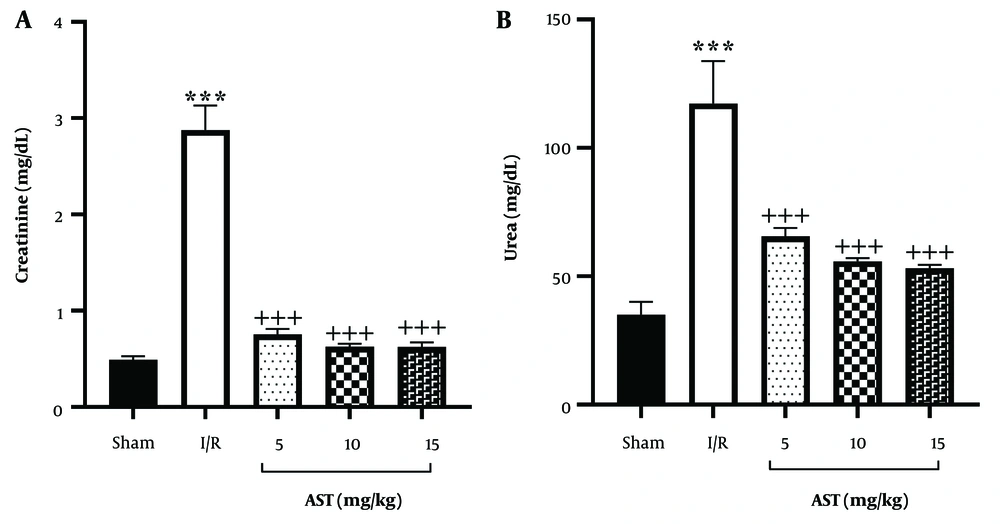
The present study revealed that 30 minutes of kidney ischemia followed by reperfusion led to a substantial rise in plasma creatinine levels in the I/R group compared to the control group (P < 0.001, Figure 1A). In contrast, administering three different doses of AST (5, 10, and 15 mg/kg) resulted in a significant reduction of plasma creatinine levels compared to the I/R group (P < 0.001). Additionally, the I/R condition was associated with a notable increase in urea levels relative to the sham group (P < 0.001, Figure 1B). However, treatment with varying doses of AST effectively lowered urea concentrations compared to the I/R group (P < 0.001).
4.2. Measurement of Serum Glutathione/Catalase Levels
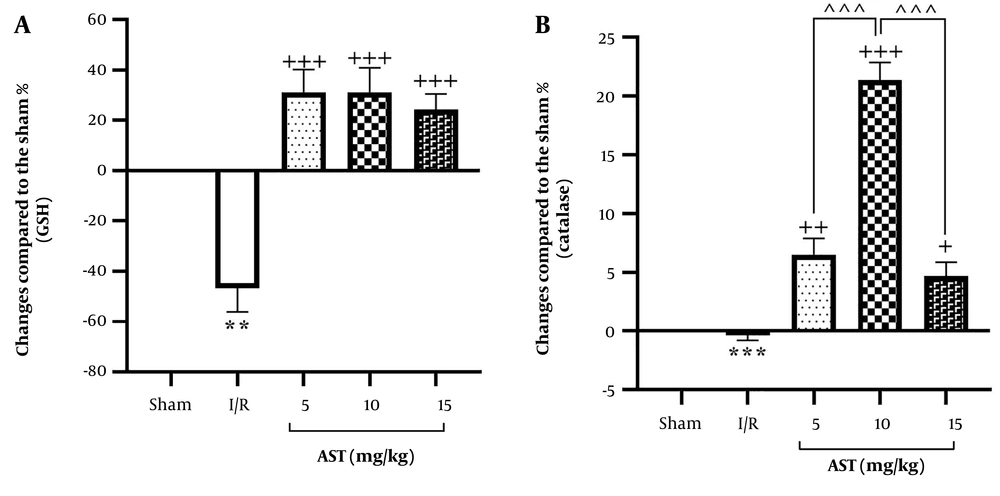
The I/R group exhibited a significant reduction in serum GSH levels compared to the sham group (P < 0.01, Figure 2A). In the treatment groups receiving AST at doses of 5, 10, and 15 mg/kg, there was a significant increase in serum GSH levels compared to the I/R group (P < 0.001). Similarly, serum catalase levels in the I/R group were markedly lower than those in the sham group (P < 0.001, Figure 2B). The administration of AST also resulted in higher serum catalase levels compared to the I/R group (P < 0.05), particularly at the 10 mg/kg dosage, which showed a highly significant difference (P < 0.001) compared to the two other doses.
Impact of AST on serum levels of GSH and catalase following renal I/R. A, GSH; and B, catalase. Data are presented as mean ± SEM. ** P < 0.01, *** P < 0.001 vs. sham; + P < 0.05, ++ P < 0.01, +++ P < 0.001 vs. I/R; ^^^ P < 0.001 vs. AST 10 mg/kg. AST, astaxanthin; GSH, glutathione; I/R, ischemia-reperfusion; SEM, standard error of the mean.
4.3. Measurement of Nitrite
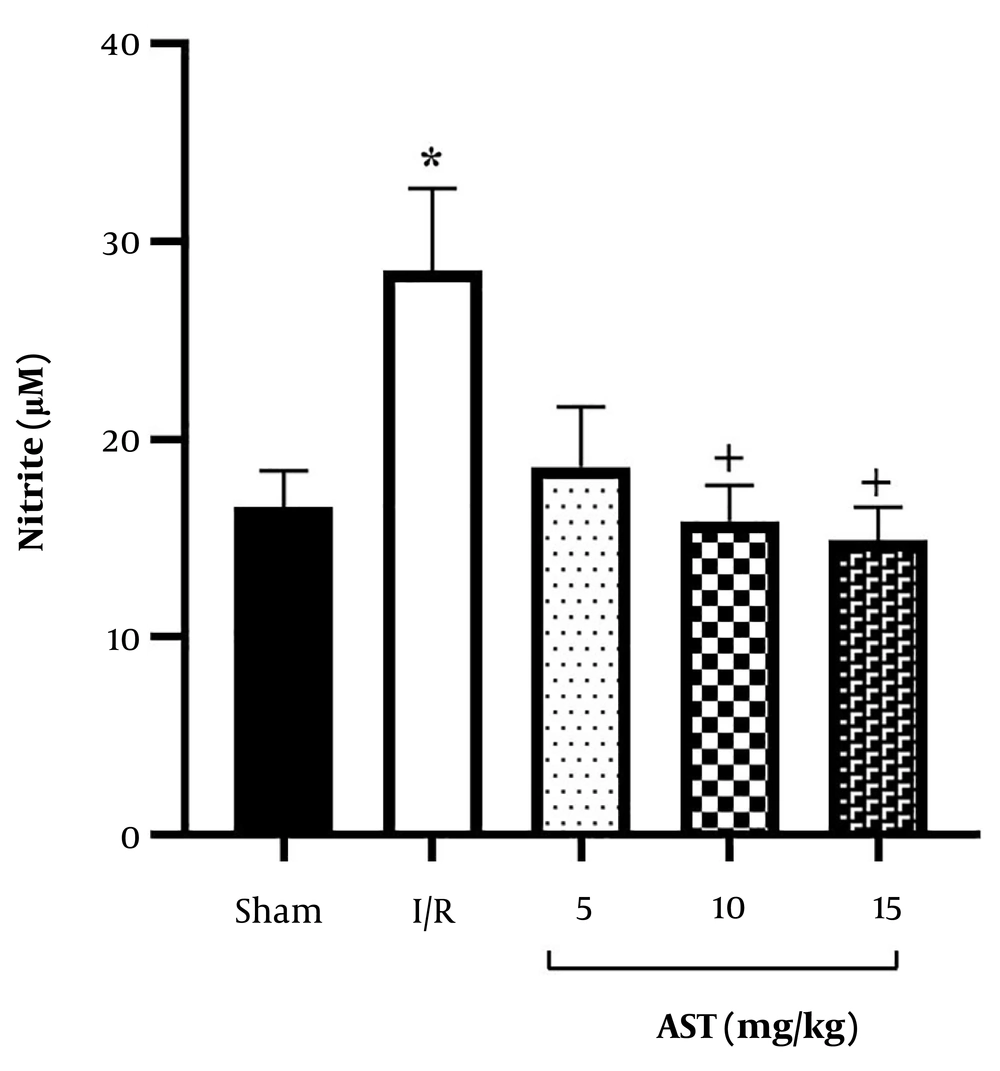
The findings indicated that I/R injury significantly elevated serum nitrite levels compared to the sham group (P < 0.05, Figure 3). Moreover, the administration of AST, particularly at doses of 10 and 15 mg/kg, resulted in a notable reduction in serum nitrite levels compared to the I/R group (P < 0.05).
4.4. Histology Changes
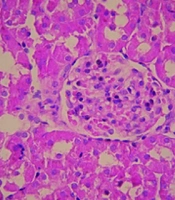
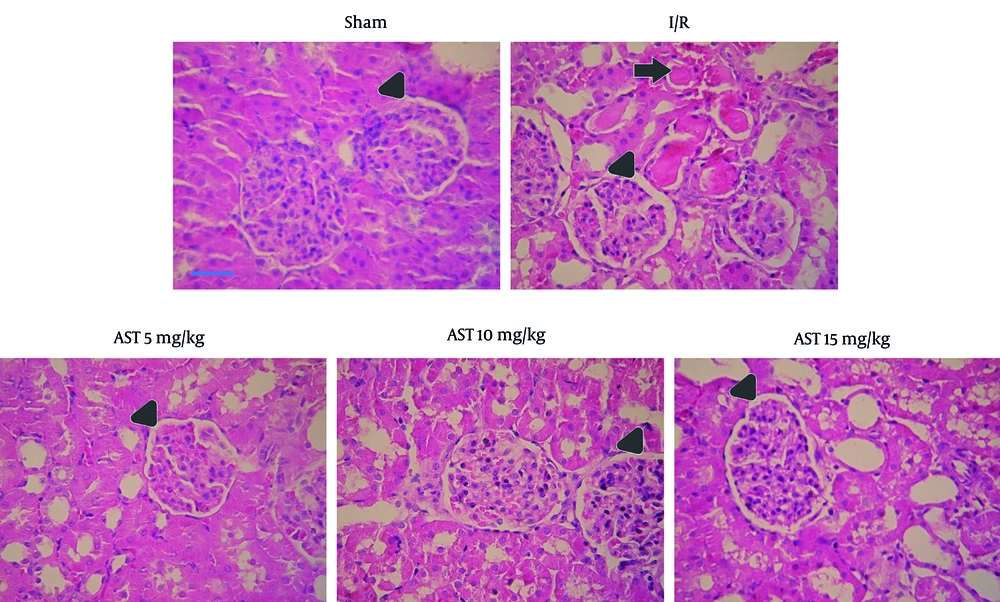
As depicted in Figure 4, I/R led to a significant increase in the size of Bowman's space within the I/R group, indicative of renal injury. In contrast, the administration of AST at doses of 5, 10, and 15 mg/kg resulted in a marked decrease in Bowman's space size compared to the I/R group. Additionally, I/R caused pronounced vascular congestion. Importantly, treatment with AST was associated with a decrease in the severity of this congestion, highlighting its potential protective effects against renal injury.
Photomicrographs of kidney sections (× 40 magnification) from sham, I/R, and AST-treated rats. The untreated group exhibited more pronounced renal injury compared to the others. Triangular arrows indicate Bowman’s space, while arrows point to regions of vascular congestion. AST, astaxanthin; I/R, ischemia-reperfusion.
5. Discussion
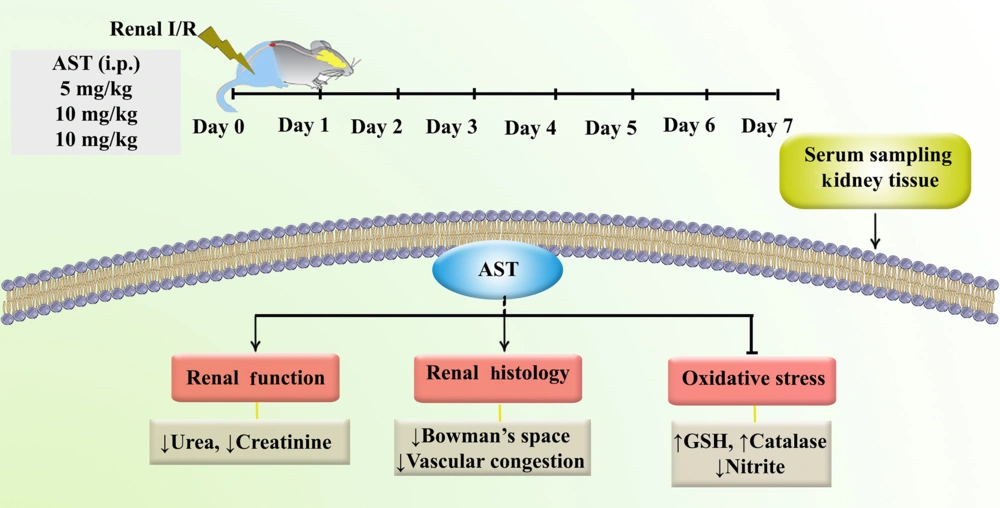
Astaxanthin showed considerable protective effects against I/R injury. Intraperitoneal administration of AST for seven consecutive days was associated with a reduction in oxidative stress markers such as nitrite and an increase in levels of key antioxidants such as GSH and catalase. This modulation helped improve kidney function and reduce tissue damage following I/R injury by regulating urea and creatinine levels (Figure 5).
Acute kidney injury is characterized by a rapid decline in kidney function, often resulting from various insults, including I/R injury. I/R occurs when the blood supply to the kidney is temporarily interrupted and then restored, leading to a cascade of pathological changes that can result in significant renal damage (16). Multiple dysregulated pathways contribute to the pathogenesis of I/R. Recent reports have introduced the canonical Wnt/β-catenin pathway as a protective mechanism, while the non-canonical Wnt pathway contributes to organ damage during I/R. The cross-talk between signaling pathways with Wnt signaling is also highlighted in controlling inflammation, oxidative stress, and apoptosis, forming a network that plays an important role in the modulation of I/R injury. Among the interconnected signaling mediators, the NF-κB, HIF-1α signaling, notch, PI3K/Akt, TGF-β, NMDA/HGF/c-Met, Rho/Rho-associated protein kinase, mitogen-activated protein kinase/extracellular signal-regulated kinase, Janus kinase/signal transducers and activators of transcription, and nuclear factor erythroid 2-related factor 2 signaling pathways deserve further investigation. Co-targeting Wnt signaling pathways and related interconnected signaling pathways offers a hopeful therapeutic candidate in I/R (2).
As a critical interconnected dysregulated pathway handled by the Wnt/β-catenin pathway, oxidative stress mediators (i.e., Nrf2, catalase, GSH, nitrite) play important roles in I/R. During the ischemic phase, there is a reduction in renal blood flow, leading to inadequate oxygen delivery to kidney tissues. This results in cellular energy depletion, primarily through the depletion of ATP, and the accumulation of metabolic waste products (17). The lack of oxygen triggers the production of ROS, which can damage cellular components, including lipids, proteins, and DNA (18). Upon restoration of blood flow, a paradoxical increase in oxidative stress occurs due to the sudden influx of oxygen. This leads to further production of ROS, exacerbating cellular injury through mechanisms such as lipid peroxidation and apoptosis. The reperfusion phase also initiates an inflammatory response characterized by the release of pro-inflammatory cytokines (e.g., tumor necrosis factor-alpha and interleukins) and chemokines, which promote leukocyte adhesion and infiltration into renal tissues (5, 19).
The main site of injury during I/R is the renal tubular epithelium. Ischemia causes tubular epithelial cell detachment, swelling, and necrosis (20). The loss of tight junction integrity allows for back-leakage of glomerular filtrate, further reducing the glomerular filtration rate. Dying cells can form obstructive casts within tubules, contributing to oliguria and worsening acute kidney injury (21, 22).
Given the complex interplay of oxidative stress and inflammatory responses in I/R injury, the protective effects of AST appear to be a promising therapeutic approach to reducing acute kidney injury. As a potent antioxidant, studies have shown that AST not only scavenges ROS but also enhances the body's endogenous antioxidant defenses (23). In our study, increased levels of GSH and catalase, as well as decreased levels of nitrite, were evident after AST administration. This property helps restore the redox balance in kidney tissues during both ischemic and reperfusion phases. Previously, we demonstrated that pretreatment with AST (5 and 10 mg/kg) delivered via solid lipid nanoparticles significantly alleviates oxidative stress, as evidenced by reduced serum nitrite levels and enhanced concentrations of critical antioxidants such as catalase and GSH in the kidneys (10). In a similar report, AST (5, 10, and 25 mg/kg) was orally used to play protective roles against renal I/R (24). We also previously showed that AST in similar doses (e.g., 5 and 10 mg/kg) exhibited anti-neuropathic potentials against chronic constriction injury of the sciatic nerve (9). In a similar range of dosages (5, 10, and 15 mg/kg), we confirmed the anti-nociceptive effects of AST through the l-arginine/nitric oxide (NO)/cyclic GMP (cGMP)/potassium channel (KATP) signaling pathway in mice (25). Our current study is the first to provide the therapeutic role of naïve AST against renal I/R in rats.
Furthermore, a separate study indicated that AST at a concentration of 250 nM effectively mitigated the decline in viability of tubular epithelial cells exposed to H2O2, underscoring its protective role against oxidative stress (26). Notably, Guo et al. reported that AST upregulates the heme oxygenase 1 axis and inhibits inflammatory pathways, such as the toll-like receptor 4 (TLR4)/MyD88/NF-κB axis, which are implicated in renal inflammation. This modulation further enhances its protective effects against acute kidney injury (27). The cumulative effects of these pathological processes lead to acute tubular necrosis, characterized by significant impairment of renal function. As previously shown (11, 28), in our study, renal I/R led to histological damage. Our results indicated that I/R caused tubular necrosis in the ascending thick portion of the Henle arc cortical areas of the proximal tubule. During I/R, we also observed an increased size of Bowman's space and numbers of red blood cells within the glomerular capillaries. In our study, AST decreased renal cortex tubule necrosis and reduced the numbers of red blood cells within the glomerular capillaries compared to the I/R group. If not promptly addressed, this can progress to chronic kidney disease or complete renal failure (29).
Our findings suggest that reducing oxidative stress is closely associated with improved kidney function, as evidenced by lower levels of key biomarkers such as creatinine and urea. Effective management of oxidative stress may play a pivotal role in safeguarding renal health and facilitating recovery following episodes of diminished blood flow and subsequent restoration, thereby alleviating tissue damage and promoting overall kidney function. Moreover, the beneficial protective effects of AST on essential indicators of renal health, including serum urea and creatinine levels, were corroborated in a mouse model. The study revealed a significant decrease in pathohistological scores, the number of apoptotic cells, and the modulation of the level of superoxide dismutase and malondialdehyde, suggesting that AST effectively reduces cellular apoptosis and fibrosis (26). We also previously showed the protective effects of a new formulation of AST (i.e., solid lipid nanoparticles) in combating kidney injury after I/R (10).
In conclusion, AST demonstrates significant protective effects against renal I/R injury in rats, evidenced by reduced renal tissue damage and improved kidney function markers such as reduced plasma creatinine and urea levels. The AST enhances antioxidant levels, including GSH and catalase, while decreasing oxidative stress markers like serum nitrite. These findings highlight AST’s potential as a therapeutic agent for acute kidney injury. Further research is needed to explore its mechanisms and clinical applicability. Additionally, more studies are needed to develop strategies that critically modulate multiple interconnected signaling mediators in I/R damage.