1. Background
Photobiomodulation (PBM), also known as low-level laser therapy (LLLT), was first identified nearly 50 years ago (1). In recent years, LLLT has gained significant traction in both medical and dental fields, primarily due to its ability to modulate biological processes without causing substantial cellular damage (2-4). Emerging evidence indicates that LLLT can modulate immune responses, including the regulation of reactive oxygen species (ROS) levels, thereby facilitating tissue repair and reducing inflammation (5-8).
The ROS are highly reactive oxygen-containing molecules that play a dual role in cellular physiology (9-11). On one hand, they are essential for cellular signaling and defense mechanisms, particularly within the immune system. On the other hand, excessive ROS production can lead to oxidative stress, contributing to cellular damage and the pathogenesis of various diseases, including cardiovascular and neurodegenerative disorders (9, 12-16).
Hamblin indicates that LLLT can lead to a temporary and modest increase in ROS production in healthy cells, resulting from an elevation of mitochondrial membrane potential (MMP) beyond normal levels (17). Nonetheless, when applied as a treatment for tissue injury or muscle damage, LLLT has been shown to effectively lower markers of oxidative stress (17-19).
Macrophages, key players in the immune response, are central to the production of ROS, which they use to combat pathogens and mediate inflammation (20, 21). Interferon-gamma (IFN-γ), a critical cytokine in both innate and adaptive immunity, primes macrophages by enhancing their capacity to produce ROS and other inflammatory mediators. As a potent macrophage activator, IFN-γ promotes the release of higher ROS levels (22, 23). However, the interaction between IFN-γ and laser irradiation, particularly its influence on ROS production, remains relatively underexplored in the current literature.
Diode lasers emitting wavelengths of 810 nm and 940 nm have been extensively studied for their capacity to penetrate tissues and influence cellular activities (3, 5, 24, 25). Given the increasing relevance of laser technology in modern dentistry and medicine, this study aims to investigate a critical and underexplored question: Do 810 nm and 940 nm diode laser irradiations differentially affect the quantitative release of ROS in macrophages, in both the presence and absence of IFN-γ? If significant differences are observed, the findings could inform the selection of optimal laser wavelengths for therapeutic applications.
This study addresses a gap in the current research landscape by examining the specific effects of 810 nm and 940 nm diode lasers on ROS production in macrophages. While previous research has demonstrated that LLLT can enhance cellular repair mechanisms, such as in the treatment of recurrent oral ulcers and scar reduction (24, 26), the direct impact of these laser wavelengths on ROS release in macrophages remains insufficiently understood and necessitates further investigation.
2. Objectives
This study aimed to compare the effects of 810 nm and 940 nm diode laser irradiation on ROS production in macrophages.
3. Methods
3.1. Cell Line and Culture Conditions
The study utilized the murine monocyte/macrophage cell line J774A.1 (NCBIC 483), acquired from the Pasteur Institute of Iran. Cells were cultured in a flask containing Dulbecco’s Modified Eagle Medium (DMEM) enriched with 10% fetal bovine serum, 100 µg/mL penicillin, 100 µg/mL streptomycin, and 2 mM L-glutamine (27, 28). The culture was maintained at 37°C in a CO2 incubator.
3.2. Laser Parameters
Two types of lasers were employed in this study: The 940 nm Epic X diode laser, featuring an adjustable power range from 0.1 to 10 W, manufactured by Biolase in the USA, and a 400 µm tip; and the 810 nm Pulsar diode laser, made in Iran, with an adjustable power range of 0.5 to 8 W, operated at 0.4 W in this experiment. Both lasers were calibrated before use by measuring their output power with a power meter.
3.3. Experimental Groups
The release of ROS by macrophage cells was evaluated in 18 different groups as presented in Table 1.
| Groups | Laser Wavelength (nm) | Power (W) | Energy (J) | Irradiation Time (s) | With/Without IFN‐γ |
|---|---|---|---|---|---|
| 1 | 810 | 0.4 | 2 | 15 | With and without |
| 2 | 810 | 0.4 | 4 | 15 | With and without |
| 3 | 810 | 0.4 | 2 | 30 | With and without |
| 4 | 810 | 0.4 | 4 | 30 | With and without |
| 5 | 940 | 0.4 | 2 | 15 | With and without |
| 6 | 940 | 0.4 | 4 | 15 | With and without |
| 7 | 940 | 0.4 | 2 | 30 | With and without |
| 8 | 940 | 0.4 | 4 | 30 | With and without |
| 9 | Control (no laser) | N/A | N/A | N/A | With and without |
Abbreviation: N/A, not applicable.
a Each group was tested twice: Once with the application of IFN‐γ and once without.
3.4. Experimental Procedure
A volume of 100 µL of the cultured J774A.1 cell line (containing 104 cells) was transferred into each well of a microplate (5 wells per group). Samples were allowed to stabilize for one hour before laser irradiation. The 940 nm and 810 nm diode lasers were employed at a 45-degree angle relative to the cell surface in the wells, using a 400 µm tip. Laser output was set at 0.4 W, with exposure durations of 15 or 30 seconds, divided into intervals with 10-second pauses in a dark environment to prevent interference from external light sources. The effective energy per well was calibrated to approximately 2 J and 4 J, corresponding to calculated energy densities of ~7 - 15 J/cm2, based on the irradiated area. These values are cumulative and not per-pulse.
Following laser irradiation, 2 µL of IFN‐γ was added to the wells to activate macrophages (29), and 4 hours post-irradiation, the ROS production level was assessed by measuring nitric oxide (NO) levels using a fluorometric method with DCFH-DA as the fluorescent probe (30, 31). Although DCFH-DA is a widely used fluorescent probe for general ROS detection, in this study, it was employed to assess NO levels as an indirect marker of ROS production. Previous research has demonstrated that DCFH-DA can respond to both ROS and reactive nitrogen species (RNS), including NO and peroxynitrite, making it suitable for detecting oxidative and nitrosative stress under specific conditions (27, 30-33).
The use of NO as a proxy for ROS has been validated in macrophage models where inducible nitric oxide synthase (iNOS) activity contributes to oxidative responses (31, 33). Nevertheless, we acknowledge that NO production can also occur independently of ROS generation, particularly via iNOS activation pathways stimulated by IFN-γ. Therefore, while our measurements reflect total oxidative activity, they may not exclusively quantify ROS.
3.5. Data Analysis
The collected data were analyzed using SPSS 24. The Kolmogorov-Smirnov test was utilized to analyze the normal distribution of data, the t-test to assess the effects of IFN-γ in each group, and the two-way analysis of variance (ANOVA) to compare ROS levels in the examined groups. A P-value < 0.05 was considered statistically significant.
4. Results
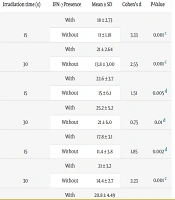
Table 2 presents a consolidated summary of NO levels under different experimental conditions, including laser wavelength, energy, exposure time, and the presence or absence of IFN-γ. The values are expressed as means ± standard deviations (SD) in micromoles (µmol). In addition to descriptive statistics, the table includes corresponding P-values and Cohen’s d effect sizes for IFN-γ comparisons to show the magnitude of biological effects.
| Laser Wavelength (nm) | Energy (J) | Irradiation Time (s) | IFN‐γ Presence | Mean ± SD | Cohen’s d | P-Value |
|---|---|---|---|---|---|---|
| 810 nm | 2 | 15 | With | 18 ± 2.73 | 3.33 | 0.001 c |
| Without | 11 ± 1.18 | |||||
| 30 | With | 21 ± 2.64 | 2.55 | 0.001 c | ||
| Without | 13.8 ± 3.00 | |||||
| 4 | 15 | With | 22.6 ± 3.7 | 1.51 | 0.005 d | |
| Without | 15 ± 6.1 | |||||
| 30 | With | 25.2 ± 5.2 | 0.75 | 0.01 d | ||
| Without | 21 ± 6.0 | |||||
| 940 nm | 2 | 15 | With | 17.8 ± 3.1 | 1.85 | 0.002 d |
| Without | 11.4 ± 3.8 | |||||
| 30 | With | 21 ± 3.2 | 2.23 | 0.001 c | ||
| Without | 14.4 ± 2.7 | |||||
| 4 | 15 | With | 20.8 ± 4.49 | 1.05 | 0.006 d | |
| Without | 15.2 ± 6.1 | |||||
| 30 | With | 24.6 ± 5.0 | 1.14 | 0.008 d | ||
| Without | 18.6 ± 5.5 | |||||
| Control (no laser) | N/A | N/A | With | 9.2 ± 0.83 | 0.66 | 0.05 e |
| Without | 8.4 ± 1.5 |
Abbreviation: SD, standard deviations.
a The mean values indicate that nitric oxide (NO) levels were consistently higher at 4 J compared to 2 J, and with 30 seconds of irradiation compared to 15 seconds. Across all conditions, the presence of IFN-γ resulted in substantially increased NO production compared to IFN-γ-absent groups. These differences were statistically significant across all laser settings (P ≤ 0.01) and were also reflected by Cohen’s d effect sizes ranging from 0.66 to 3.33, indicating moderate to very large biological effects of IFN-γ.
b Laser treatment at both 810 nm and 940 nm, with varying energy levels and exposure times, significantly enhanced NO production when combined with IFN-γ stimulation compared to both IFN-γ-absent groups and untreated controls. The control group also demonstrated a significant difference between IFN-γ-stimulated and unstimulated conditions (P = 0.05, Cohen’s d = 0.66), supporting the role of IFN-γ in promoting oxidative activity even in the absence of photostimulation.
c P ≤ 0.001.
d P ≤ 0.01.
e P ≤ 0.05.
Cohen’s d values were calculated for each condition to quantify the magnitude of IFN-γ's effect on NO levels. The results demonstrate consistently large effect sizes (d > 0.8), with several conditions exceeding d = 2.0, indicating a very large stimulatory effect of IFN-γ on ROS production as measured by NO.
Based on the Kolmogorov-Smirnov test, data were normally distributed (P = 0.390), and Levene's test for equality of variances resulted in a P-value of 0.82, confirming that the variances are homogeneous.
One-way ANOVA revealed that irradiation energy (F = 19.00, P = 0.001), irradiation time (F = 12.20, P = 0.001), and the presence of IFN-γ (F = 40.60, P < 0.001) all had significant main effects on NO production. In contrast, laser wavelength (810 nm vs. 940 nm) showed no significant effect (F = 0.22, P = 0.63). Additionally, no two-way, three-way, or four-way interaction terms were statistically significant (all P > 0.05), suggesting that these factors act independently rather than synergistically in modulating NO levels.
5. Discussion
This study aimed to evaluate the effect of two diode laser wavelengths, 810 nm and 940 nm, on ROS in macrophage cells, using NO levels as an indirect but widely accepted proxy for total oxidative activity. We hypothesized that the two wavelengths might influence ROS production differently due to their distinct absorption profiles. Additionally, we assessed how variations in irradiation energy, irradiation time, and the presence of IFN-γ as a stimulant influenced ROS release. A control group, not exposed to laser irradiation, was included to provide a baseline for comparison.
Our results demonstrated that both 810 nm and 940 nm diode lasers can significantly increase ROS production compared to the control group. However, no significant difference was observed in ROS release between the two wavelengths, leading to the rejection of the null hypothesis. Additionally, we observed that higher irradiation energy (4 J versus 2 J) and longer irradiation times (30 seconds versus 15 seconds) substantially elevated NO levels, indicating a corresponding increase in ROS production.
These results align with previous studies like Cerdeira et al(34) and Ivanov et al. (31). Cerdeira et al.(34) found that LLLT can increase ROS production in neutrophils. Ivanov et al. (31) identified a positive correlation between ROS production and irradiation time but did not examine energy density (31). Our study builds on this by demonstrating that higher energy density also leads to increased ROS production, aligning with the results of Chen et al. (35).
In contrast to our findings, da Silva Neto Trajano et al. (36) observed no significant difference in ROS production between laser-irradiated groups and the control group. Similarly, Yin et al. (37) reported a reduction in ROS levels with a 660 nm diode laser compared to their control, which is contrary to our results showing increased ROS levels with both 810 nm and 940 nm lasers. These discrepancies may be attributed to differences in experimental conditions, such as laser wavelength, power, energy density, and the use of IFN-γ as a stimulant in our study.
Our study extends existing research by comparing two different diode laser wavelengths (810 nm and 940 nm). Previous studies, such as those by Yin et al. (37) and Chen et al. (35), primarily focused on single wavelengths without directly comparing different wavelengths as we did. The results of the current study also revealed that IFN-γ led to a significant increase in ROS levels, indicating its efficacy in stimulating macrophage activation. The IFN-γ is an important cytokine in both innate and adaptive immune responses, and it enhances the secretion of anti-inflammatory cytokines by macrophages (29, 30).
While no other studies have used IFN-γ to stimulate ROS production, our findings show a significant increase in ROS levels with its use. Compared to other priming agents such as LPS (31, 37), IFN-γ appears to elicit a distinct and possibly more sustained activation of ROS-generating pathways. Ivanov et al. (31) demonstrated that LPS-stimulated serum proteins generate long-lived ROS species following laser exposure, whereas IFN-γ may more directly and robustly activate intracellular enzymatic machinery. In fact, the observed synergistic effect between IFN-γ and laser irradiation in enhancing ROS production may be attributed to the activation of multiple ROS-generating pathways. The IFN-γ is known to prime macrophages through the upregulation of NADPH oxidase, an enzyme complex responsible for producing superoxide radicals during the respiratory burst (20, 29, 38). Simultaneously, laser irradiation, particularly in the near-infrared range, can stimulate mitochondrial ROS production via cytochrome c oxidase (CCO) activation. The combined stimulation of mitochondrial and NADPH oxidase pathways likely accounts for the significantly elevated ROS levels observed in IFN-γ-treated, laser-irradiated groups. This dual-pathway activation supports the hypothesis that IFN-γ sensitizes macrophages to LLLT by amplifying oxidative responses.
The absorption of light by mitochondrial chromophores, particularly CCO, is significant due to its prominent absorption peaks in the near-infrared region, especially around 810 nm (12). While we hypothesized that 810 nm would stimulate ROS production more effectively due to stronger CCO absorption, our findings showed no significant difference between the 810 nm and 940 nm wavelengths. This suggests that, although 940 nm is outside CCO’s peak absorption range, it may still exert biological effects via secondary pathways such as water absorption or less well-characterized mitochondrial components. These results highlight the complexity of wavelength-specific interactions and underscore the need for further mechanistic studies.
While NO was used as a surrogate marker of ROS in this study, it is important to acknowledge that NO production can occur independently via iNOS activity and may not fully capture the complexity of ROS. Nonetheless, DCFH-DA is a validated probe for detecting general oxidative and nitrosative stress, including species such as NO, hydrogen peroxide, and peroxynitrite (27, 31). To improve specificity, future studies should incorporate direct ROS detection methods, such as dihydroethidium (DHE) for superoxide and Amplex Red for hydrogen peroxide, to obtain a more nuanced profile of oxidative changes in macrophages post-irradiation.
The ROS are central to many physiological and pathological processes, ranging from microbial defense and wound healing to chronic inflammation and carcinogenesis (39, 40). The ability to modulate ROS levels via laser therapy has potential clinical applications in tissue regeneration, immune modulation, and inflammation control (39-41). Given ROS’s dual role, being both beneficial and potentially harmful, precise control of laser parameters (wavelength, energy density, duration) is essential to ensure therapeutic safety and efficacy (42, 43).
While our study provides valuable insights, it is important to acknowledge its limitations. This study was conducted on a specific type of macrophage cell, and the results may not be generalizable to other cell types or in vivo conditions. The J774A.1 murine macrophage cell line was selected due to its reproducibility, availability, and established use in oxidative stress and immunomodulation studies. While this model provides consistency for controlled in vitro experimentation, it may not fully reflect the complexity of primary macrophages derived from human tissue. Differences in receptor expression, mitochondrial function, and cytokine responsiveness between murine cell lines and primary human macrophages could influence ROS production. Therefore, caution should be exercised when extrapolating these results to human physiology, and future studies should validate these findings using primary macrophages or human-derived cell models.
Future studies should aim to replicate these findings in different cell lines and explore the effects of other laser wavelengths and energy densities. Specifically, future studies should investigate the relationship between LLLT, ROS production, and clinical outcomes. Additionally, more extensive cellular studies are suggested to better understand the underlying mechanisms of laser-induced ROS production and its therapeutic potential.
Temperature changes during laser exposure were not continuously monitored in this study. However, the low-level irradiation parameters used (0.4 W for a maximum of 30 seconds) fall within established non-thermal LLLT thresholds (3, 25). Previous studies have shown that under such conditions, cellular temperature elevations are minimal and unlikely to contribute significantly to the observed biological effects. Nevertheless, future work should incorporate direct temperature monitoring to definitively exclude thermal contributions to ROS generation.
In addition, future work should investigate how the mode of laser emission, pulsed versus continuous wave, modulates ROS production. Pulsed lasers may reduce heat buildup while still stimulating mitochondrial chromophores efficiently, potentially resulting in distinct oxidative responses compared to continuous wave modes. Some studies suggest that pulsed irradiation may produce lower peak ROS levels or induce different signaling cascades. Exploring this variable will be critical for optimizing LLLT protocols for specific clinical applications.
5.1. Conclusions
In conclusion, our study demonstrates that while the specific diode laser wavelengths of 810 nm and 940 nm do not significantly differ in ROS release, both significantly increase ROS production compared to a control group. Furthermore, irradiation time, energy density, and the presence of IFN-γ play crucial roles in modulating ROS production in macrophage cells.