1. Background
Major depression (MD) is a serious mental health issue worldwide, affecting approximately 4.4% of the global population (1). The primary symptoms of MD include feelings of sadness, loss of interest, reduced appetite, and thoughts of self-harm or suicide (2). The pathophysiology of MD is not yet fully understood. However, one of the earliest theories is the monoamine hypothesis, which suggests that depression symptoms result from alterations in monoamine levels, including serotonin (5-HT), noradrenaline (NA), and dopamine (DA) in the nervous system (3). Moreover, elevated levels of oxidative stress markers and a decreased ability of the body to combat oxidative damage with antioxidants play a key role in the pathogenesis of MD (4, 5).
On the other hand, a new concept of antidepressant activity has been proposed based on growing evidence illustrating the antioxidant effects of antidepressants [e.g., fluoxetine (Flx)] in the treatment of MD (4, 5). The enhancement of antioxidant defenses may be one of the mechanisms underlying the neuroprotective activity of several antidepressants in the treatment of MD (4, 5). Although there are many treatment options available for MD, the common side effects of antidepressants compel researchers to seek safer or more tolerable agents. In recent years, there has been increasing interest in using essential oils for their potential to address various health issues, including MD (6, 7). Due to their small, fat-soluble components, essential oils can easily cross the blood-brain barrier to reach brain tissue, where they interact with the thalamus, cerebral cortex, and limbic system (8). Additionally, the use of antioxidant agents, such as herbal essential oils, has been associated with improvements in MD and could potentially be used alongside antidepressant treatments to enhance their effects (6, 8).
Heracleum persicum, commonly known as Golpar, is a flowering plant from the Apiaceae family, native to Asian countries such as Iran. It has been traditionally used to treat various conditions, including neurological diseases (9). Heracleum persicum contains various classes of natural compounds, comprising both volatile and non-volatile compounds (10). Several studies have reported the antioxidant properties of H. persicum (11, 12) and its effectiveness in treating neurological diseases (9, 13). Supporting this view, a study demonstrated that at least some of the anti-inflammatory effects of the essential oil and hydroalcoholic extract of H. persicum fruits may be attributed to its antioxidant constituents, particularly furanocoumarins (14). Notably, furanocoumarins themselves also exhibit antidepressant-like effects (15). Considering the involvement of oxidative stress in the pathogenesis of MD and the potential role of H. persicum components (e.g., furanocoumarins) in depression, we aimed to investigate the antidepressant potential of H. persicum essential oil (HPEO) and its possible mechanisms.
2. Objectives
No study has been conducted to evaluate the antidepressant potential of H. persicum. Therefore, this research was undertaken to assess the antidepressant potential of HPEO in the forced swim test (FST) in mice by investigating its antioxidant and monoaminergic mechanisms.
3. Methods
3.1. Preparation of Heracleum persicum Essential Oil
Heracleum persicum was obtained from the Diar Zagros Health Center (Kermanshah, Iran) and identified by botanists at the Herbarium of the Medicinal Plants Research Institute (Karaj, Iran) (OTH-408-1125). The essential oil was extracted using water distillation with a Clevenger apparatus. A total of 300 g of the sample was separately crushed using a mixer. The powdered fruits were then placed in a two-liter flask of the device along with 700 mL of distilled water. The mixture was boiled for one and a half hours, after which the essential oil was separated from the water (16).
3.2. Gas Chromatography-Mass Spectrometry (GC-MS)
The analysis was conducted using a 5975, 7890 model device (Agilent, USA) equipped with an MS detector and an H5-5MS capillary data processor (DB-30 m × 0.25 mm, i.d.; 0.25 μm film thickness). The injector temperature was set at 260°C. The identification of essential oil compounds was performed using the Kovats Retention Index (KI) and Wiley Library guidelines (17).
3.3. Drugs or Chemicals
The drugs used in this study were as follows: Fluoxetine, SCH23390, WAY100135, yohimbine, ketanserin, pCPA, haloperidol, reserpine, prazosin, sulpiride, and yohimbine. All agents were procured from Sigma Co. (USA). The drugs were administered intraperitoneally (i.p.) and dissolved in 2% tween 80.
3.4. Animals
Male Swiss mice (22 - 28 g) were used in this study. The animals were purchased from Danesh Pajoohan Hirad Darou Co. (Kermanshah, Iran) and were maintained under standard laboratory conditions, including a temperature of 22 ± 2°C, a 12-hour light/dark cycle, 55% humidity, and were fed commercial food pellets and water, except during the testing periods.
3.5. Forced Swim Test
In this test, the mice were individually placed in a 5-liter container (8 × 12 × 25 cm) filled with water at 25°C. The total test duration was 6 minutes, with the first 2 minutes allotted for the animal to acclimate to the environment. During the remaining 4 minutes, the time spent immobile was recorded using a chronometer. In this test, a reduction in immobility time was recorded as an antidepressant-like effect (18).
3.6. Open Field Test
To minimize animal usage, the motor activity of the mice was assessed in the open field test (OFT) 5 minutes before starting the FST. The box was made of Plexiglass with dimensions of 35 × 45 × 45 cm and divided into 9 square sections (15 × 15 cm each) by marked lines. Movement from one section to another using all four limbs was considered one unit of movement. The total number of movements made by each mouse in the box during the 5 minutes was recorded using a counter (19).
3.7. Grouping
In this study, 72 mice were divided into 12 groups. Each group consisted of 6 animals and received the following treatments:
Group 1: Normal saline with 2% tween 80 as a vehicle or negative control.
Group 2: The Flx as the positive control (20 mg/kg).
Groups 3 - 5: Different doses of HPEO (125, 250, and 500 mg/kg).
In this section, 25 minutes after the injection of the essential oil, the OFT was conducted, and immediately afterward, the same mice were transferred to the FST for further evaluation.
3.8. Possible Mechanisms
In this part, one hour after receiving the various dopaminergic, serotonergic, and noradrenergic antagonists, the effective dose of HPEO (500 mg/kg) was administered, and after 30 minutes, the mice were subjected to the FST.
3.8.1. The Role of the Dopaminergic System
Groups 6 - 7 Received SCH23390 (0.05 mg/kg, D1 DA receptor antagonist), sulpiride (50 mg/kg, D2 DA receptor antagonist), and haloperidol (0.2 mg/kg, non-selective DA receptor antagonist) (13) along with 500 mg/kg of HPEO.
3.8.2. The Role of the Noradrenergic System
Groups 8 and 9 Received prazosin (1 mg/kg, α1 adrenoceptor receptor antagonist) and yohimbine (1 mg/kg, α2 adrenoceptor receptor antagonist) (20) along with 500 mg/kg of HPEO.
3.8.3. The Role of the Serotonergic System
Groups 10 - 11 Received WAY100135 (10 mg/kg, 5-HT1A/1B receptor antagonist) and ketanserin (5 mg/kg, 5-HT2A/2C receptor antagonist) (22) along with 500 mg/kg of HPEO.
Moreover, group 12 received pCPA (150 mg/kg, 5-HT synthesis inhibitor) for three consecutive days at specific times, and 24 hours after the last dose of pCPA, they received 500 mg/kg of HPEO and were then subjected to testing.
In this work, the doses of HPEO (21, 22) and drugs were chosen based on the literature and our earlier research (20).
3.9. Measurement of Antioxidant Activity
In this study, antioxidant activity was measured using kits from Kiyazist (Hamadan, Iran) to assess nitric oxide (NO), catalase (CAT), and glutathione (GSH) levels. After anesthetizing the mice with thiopental (45 mg/kg), blood was collected from the heart. The sample was incubated at 37°C for 15 minutes, centrifuged at 4,000 rpm for 15 minutes, and the mouse serum was stored at -80°C for further enzyme studies (23).
3.10. Data Analysis
The results are presented as mean ± SEM The data were analyzed using one or two way ANOVA followed by the Tukey test. GraphPad Prism version 9 (USA) was used for analysis and graph plotting. The significance level was set at P < 0.05.
4. Results
4.1. Chemical Components of Heracleum persicum Essential Oil
The GC/MS analysis revealed that the major compounds in HPEO included hexyl butanoate (33.96%), acetic acid (23.48%), butanoic acid (8.18%), butanoic acid 2-methyl octyl ester (5.30%), hexyl hexanoate (3.15%), and propanoic acid (2.95%). The remaining compounds were present in concentrations below 2% (Table 1).
| No. | Components | RT (min) | KI | Percentage (%) |
|---|---|---|---|---|
| 1 | Propanoic acid, 2-methyl-, 1-methylethyl ester | 5.45 | 794 | 0.93 |
| 2 | Hexanol < n- > | 8.47 | 870 | 0.28 |
| 3 | Isopropyl 2-methylbutyrate | 9.02 | 885 | 1.05 |
| 4 | Isopropyl 3-methylbutanoate | 9.42 | 904 | 1.33 |
| 5 | Isobutyl isobutyrate | 12.33 | 911 | 0.25 |
| 6 | Butyl butanoate | 14.61 | 994 | 0.61 |
| 7 | Octanal < n- > | 15.14 | 998 | 0.65 |
| 8 | Hexyl acetate | 15.48 | 1009 | 0.44 |
| 9 | Cymene | 16.12 | 1029 | 0.69 |
| 10 | Butyl 2-methylbutanoate | 16.89 | 1038 | 0.37 |
| 11 | Butanoic acid, 3-methyl-, butyl ester | 17.23 | 1054 | 0.29 |
| 12 | γ-Terpinene | 17.81 | 1060 | 0.93 |
| 13 | Octanol < n- > | 18.61 | 1068 | 1.19 |
| 14 | Terpinolene | 20.03 | 1088 | 0.58 |
| 15 | Propanoic acid, 2-methyl-, hexyl ester | 22.30 | 1150 | 1.63 |
| 16 | Hexyl butanoate | 24.57 | 1192 | 33.96 |
| 17 | Cyclohexane, ethenyl- | 24.81 | 1197 | 3.26 |
| 18 | Acetic acid, octyl ester | 25.48 | 1213 | 23.48 |
| 19 | Hexyl 2-methyl butanoate < n- > | 26.58 | 1236 | 2.76 |
| 20 | Hexyl isovalerate | 26.90 | 1244 | 0.40 |
| 21 | Anethole < (E)- > | 29.43 | 1284 | 0.84 |
| 22 | Propanoic acid, 2-methyl-, octyl ester | 31.53 | 1348 | 2.95 |
| 23 | Hexyl hexanoate | 33.41 | 1383 | 3.15 |
| 24 | Butanoic acid, octyl ester | 33.57 | 1394 | 8.18 |
| 25 | Butanoic acid, 2-methyl-, octyl ester | 35.37 | 1436 | 5.30 |
| 26 | Butanoic acid, 3-methyl-, octyl ester | 35.64 | 1442 | 0.58 |
| Total identified | 96.08 | |||
Abbreviations: RT, retention time; KI, Kovats Index.
4.2. Behavioral Responses
4.2.1. Effects of Heracleum persicum Essential Oil on Immobility Time in Forced Swim Test
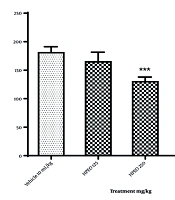
As shown in Figure 1, both 250 mg/kg and 500 mg/kg doses of HPEO, as well as Flx, significantly reduced immobility time compared with the vehicle (control) group (P < 0.001). The 250 mg/kg dose of HPEO was less effective than the 500 mg/kg dose, which significantly reduced immobility time (P < 0.001). The effect of the high dose (500 mg/kg) of HPEO was comparable to that of Flx, with no significant difference observed between them (P = 0.6839). Since the 500 mg/kg dose of HPEO demonstrated the greatest reduction in immobility behavior in the FST, this dose was selected to investigate the mechanism of action.
4.2.2. Effects of Heracleum persicum Essential Oil on Animal Locomotion in Open Field Test
As displayed in Table 2, all doses (125, 250, and 500 mg/kg) of HPEO did not significantly change the number of square crossings (P = 0.2485, 0.2585, and P = 0.9015, respectively) or rearing behavior (P = 0.2538, 0.1674, and P = 0.0842, respectively) compared to the vehicle group.
| Groups | Dose | Number of Square Crossings | Number of Rearing |
|---|---|---|---|
| Vehicle (control); mL/kg | 10 | 56.0 ± 1.98 | 17.00 ± 1.24 |
| HPEO; mg/kg | 125 | 60.00 ± 3.07 | 15.70 ± 1.36 |
| HPEO; mg/kg | 250 | 61.50 ± 2.23 | 18.70 ± 0.76 |
| HPEO; mg/kg | 500 | 65.30 ± 4.18 | 20.00 ± 0.57 |
Abbreviation: HEPO, Heracleum persicum essential oil.
a Results are expressed as mean ± SEM (n = 6).
4.3. Role of the Dopaminergic System in the Antidepressant Mechanism of Heracleum persicum Essential Oil in the Forced Swim Test
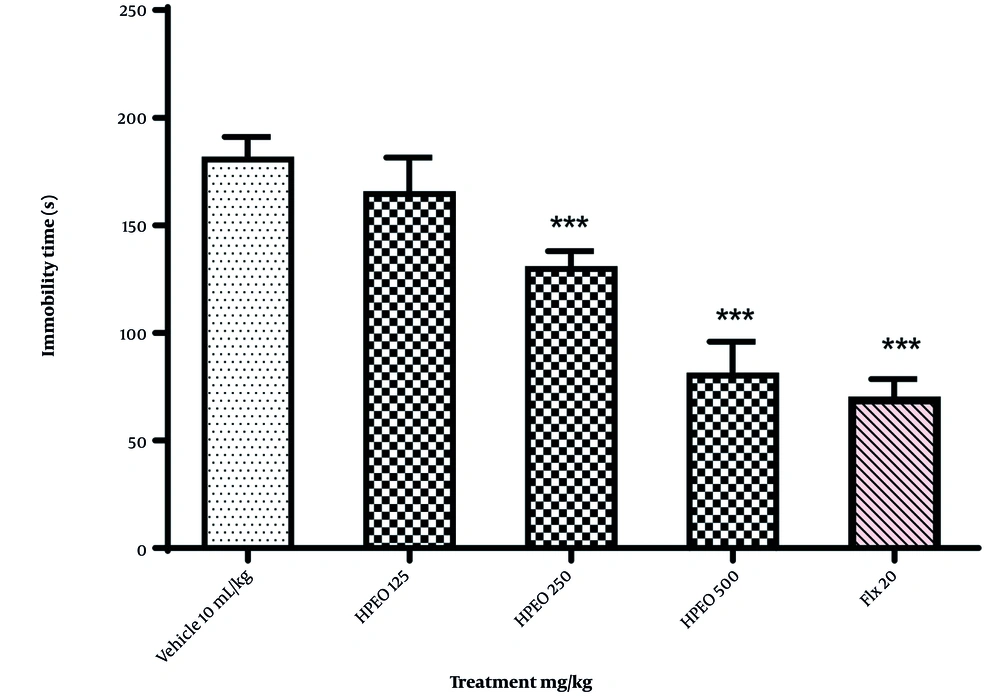
Pretreatment with sulpiride, SCH23390, and haloperidol one hour before the administration of HPEO prevented the antidepressant effect of HPEO (500 mg/kg) (Figure 2).
Pretreatment with SCH23390 (0.05 mg/kg; D1 receptor antagonist), sulpiride (50 mg/kg; D2 receptor antagonist), and haloperidol (0.2 mg/kg; non-selective dopamine (DA) receptor antagonist) on the antidepressant potential Heracleum persicum essential oil (HPEO) in the forced swim test (FST). The data are presented as mean ± SEM (n = 6). *** P < 0.001 versus the vehicle group
4.4. Role of the Noradrenergic System in the Antidepressant Mechanism of Heracleum persicum Essential Oil in Forced Swim Test
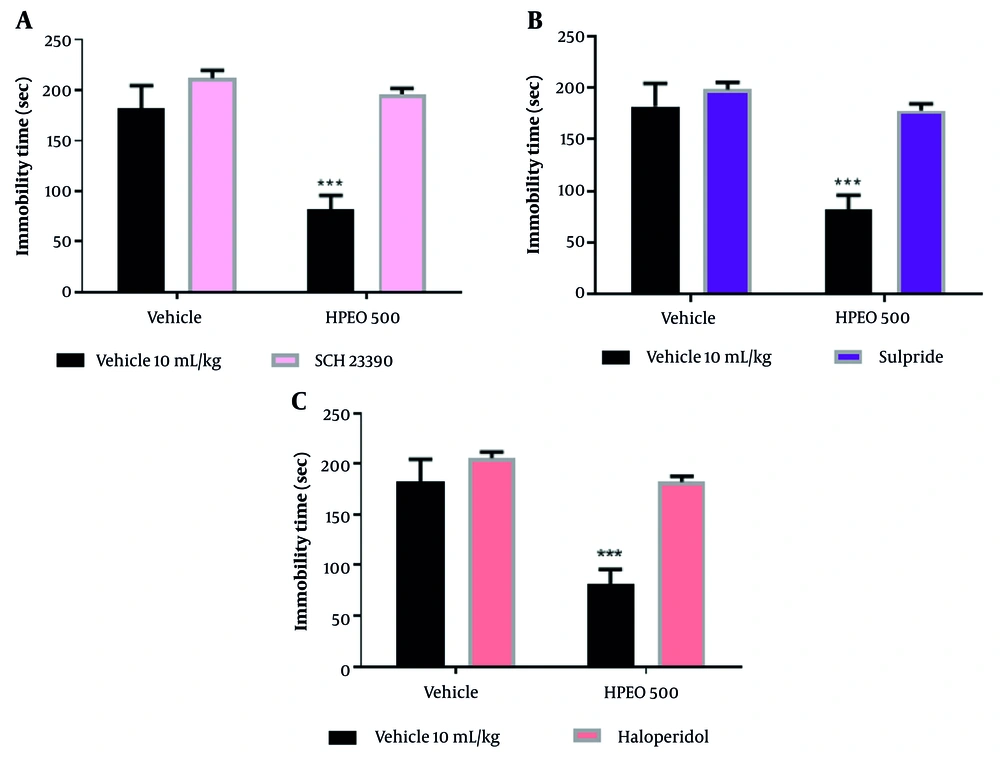
Pretreatment with yohimbine and prazosin one hour before the administration of HPEO prevented the antidepressant potential of HPEO (500 mg/kg) (Figure 3).
The pre-treatment with prazosin (1 mg/kg; α1 receptor antagonist), yohimbine (1 mg/kg; α2 receptor antagonist) on the antidepressant potential Heracleum persicum essential oil (HPEO) in the forced swim test (FST). The data are presented as mean ± SEM (n = 6). *** P < 0.001 versus the vehicle group.
4.5. Role of the Serotonergic System in the Antidepressant Mechanism of Heracleum persicum Essential Oil in Forced Swim Test
Pretreatment with WAY100135, pCPA, and ketanserin one hour before administering HPEO (500 mg/kg) prevented the antidepressant potential of HPEO (Figure 4).
Pretreatment with WAY100135 (5-HT1A/1B receptor antagonist, 10 mg/kg), ketanserin (5-HT2A serotonergic receptor antagonist, 5 mg/kg), and p-chlorophenylalanine (pCPA) [serotonin (5-HT) synthesis inhibitor, 150 mg/kg] on the antidepressant potential of Heracleum persicum essential oil (HPEO) in the forced swim test (FST). Results are presented as mean ± SEM (n = 6). + p < 0.05 versus the HPEO group (500 mg/kg). *** P < 0.001 versus the vehicle group.
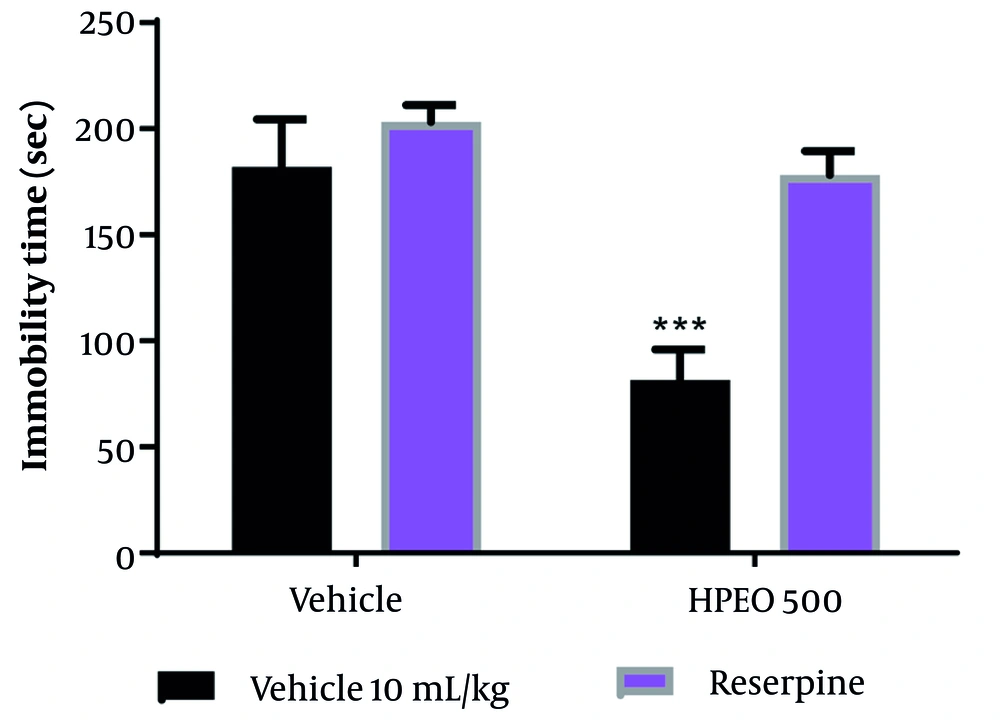
4.6. Pretreatment with Reserpine in Forced Swim Test
Pretreatment with reserpine four hours before administering HPEO (500 mg/kg) prevented the antidepressant potential of HPEO (Figure 5).
4.7. Antioxidant Activity
The results indicated that all groups receiving HPEO at doses of 125 to 500 mg/kg (P < 0.05 and P < 0.01, respectively) and Flx (P < 0.01) exhibited significant increases in serum GSH activity (Figure 6). However, Flx was significantly more effective than the different doses of HPEO (P < 0.05). Additionally, HPEO at doses of 250 and 500 mg/kg and Flx (P < 0.01) increased CAT levels (P < 0.05 and P < 0.01, respectively). Both the HPEO and Flx groups significantly decreased NO levels (P < 0.01 and P < 0.001, respectively).
Effects of Heracleum persicum essential oil (HPEO) on the serum antioxidant enzyme levels [glutathione (GSH), catalase (CAT) and nitric oxide (NO)]. The data are presented as mean ± SEM (n = 6). * P < 0.05, ** P < 0.01 and *** P < 0.001 versus the vehicle group; # P < 0.05 and ## P < 0.01 versus the fluoxetine (Flx) group
5. Discussion
The findings revealed that hexyl butanoate, acetic acid, butanoic acid, butanoic acid 2-methyl octyl ester, hexyl hexanoate, and propanoic acid were major compounds in HPEO. Previous studies have shown that the common phytochemical compounds in HPEO are volatile compounds, terpenoids, triterpenes, flavonoids, and alkaloids (9, 10). Another study reported that anethole was the primary compound in the oils extracted from the leaves, flowers, and stems of HPEO. Therefore, monoterpenes such as anethole, limonene, and terpinolene are also considered significant constituents of this essential oil (24). With that in mind, previous studies demonstrate the antidepressant potential of monoterpenes via modulation of the monoaminergic system (9, 25, 26). Furthermore, furanocoumarins are the other main components found in HPEO (9). In this regard, a recent study showed the antidepressant potential of furanocoumarins via modulation of monoamine oxidase-B (MAO-B) inhibition and their inhibitory effect on neurotransmitter or monoaminergic pathways (15).
The findings of the current work illustrated that the 250 and 500 mg/kg doses of HPEO reduced immobility time in the mouse FST. Notably, the antidepressant effect of the 500 mg/kg dose was comparable to that of Flx. Consistent with our findings, previous studies have reported that antidepressant drugs reduce immobility time in both behavioral models, indicating an antidepressant-like effect (20, 26). The FST, in particular, is widely used to evaluate various antidepressants, including Flx (18). Additionally, our results demonstrated that HPEO did not cause significant changes in locomotor activity, as assessed by the OFT. The results were confirmed by the absence of notable differences in the number of square crossings or rearing behaviors. Importantly, increased locomotion in the OFT is not desirable, as it may lead to false-positive antidepressant effects (27).
Our findings demonstrated that various DA receptor antagonists, including sulpiride, SCH23390, and haloperidol, significantly reversed the antidepressant potential of HPEO (500 mg/kg). These findings are consistent with earlier studies (9, 25, 28). Notably, no prior research has investigated the dopaminergic effects of HPEO. Previous studies have shown that DA levels are decreased in depressed individuals (29). Therefore, drugs with dopaminergic mechanisms can be effective and beneficial in the treatment of depression (30). In line with this, the administration of Flx has been reported to increase DA levels in the nucleus accumbens (31). These findings support the efficacy of HPEO for the management of depression through the dopaminergic system.
Parallel to the role of the dopaminergic system, the noradrenergic system also plays an important role in the regulation of depression and the pharmacologic effects of most antidepressant drugs (32). The findings of the current work show that different adrenoceptor receptor antagonists, including yohimbine and prazosin, abolished the antidepressant potential of HPEO (500 mg/kg). Some common antidepressants, such as venlafaxine, increase NA levels in the synaptic cleft (25). Therefore, based on the results of the current work, a part of the antidepressant effects of HPEO may be related to its interaction with the noradrenergic system.
The results of the current study illustrated that pretreatment with a 5-HT synthesis inhibitor (pCPA) and different 5-HT receptor antagonists, including ketanserin and WAY100135, significantly reversed the antidepressant potential of HPEO (500 mg/kg). This suggests that the serotonergic mechanism plays an important role in mediating the antidepressant potential of HPEO. Various 5-HT receptors, such as 5-HT1A and 5-HT2, play a crucial role in the mechanism of antidepressant agents (31). Supporting this hypothesis, blocking these receptors has been shown to inhibit the antidepressant potential of certain medicinal plants and plant-derived compounds in animal models of depression (20, 26, 28). The results confirm the involvement of HPEO in depression via the serotonergic system.
Our results also showed that pretreatment with the vesicular monoamine depletor reserpine blocked the antidepressant potential of HPEO (500 mg/kg) in the FST. This finding suggests that HPEO interacts with the monoaminergic system. Thus, HPEO may modulate brain monoamines, contributing to its antidepressant effects. In summary, HPEO exerts its effects through the monoaminergic system in depression.
A part of the antidepressant mechanism of HPEO could be attributed to its antioxidant properties. Antioxidants such as CAT, GSH peroxidase, and NO help mitigate oxidative stress, which is often elevated in MD, further influencing the disorder's pathophysiology (33). Elevated levels of NO are associated with increased oxidative damage (34), while antioxidant systems like CAT and GSH help regulate this balance, potentially affecting the severity of depressive symptoms. In this regard, patients who suffer from MD show lower serum/plasmatic total antioxidant potentials and reduced brain GSH levels compared to normal controls (35, 36). Additionally, antioxidants protect the monoaminergic system and prevent its damage (33-36).
In the current study, hexyl butanoate, acetic acid, butanoic acid, butanoic acid 2-methyl octyl ester, hexyl hexanoate, and propanoic acid were identified as major compounds with antioxidant properties (37). Thus, HPEO contains antioxidant compounds that protect the monoaminergic system.
However, this work had certain limitations. Firstly, while we observed the acute antidepressant potential of HPEO in the FST, it is necessary to test other mouse models of depression (e.g., chronic mild stress) to evaluate its chronic efficacy. Secondly, although we demonstrated the role of brain monoamines and antioxidant defense in the antidepressant mechanism of HPEO, it is necessary to investigate other mechanisms (e.g., the role of inflammatory mediators) in the antidepressant potential of HPEO. Thirdly, our work suggests that HPEO has antidepressant potential. Therefore, it is important to evaluate and identify the role of its active components in its antidepressant activities. Finally, as the current study was performed on mice, the findings cannot be easily generalized to humans. Hence, human clinical trials are required to determine the safety and effectiveness of HPEO in the management or treatment of MD.
5.1. Conclusions
Our findings revealed the antidepressant effect of HPEO. Based on the results, its antidepressant effects are mediated through the monoaminergic system, specifically involving dopaminergic, serotonergic, and noradrenergic systems, as well as antioxidant mechanisms. However, our results are preliminary, and further investigations are suggested to elucidate the safety profile and determine which isolated compounds are responsible for HPEO's antidepressant potential. Furthermore, additional studies (e.g., clinical trials) are needed to explore the potential use of HPEO for controlling and treating depression in humans.
![Pretreatment with WAY100135 (5-HT1A/1B receptor antagonist, 10 mg/kg), ketanserin (5-HT2A serotonergic receptor antagonist, 5 mg/kg), and p-chlorophenylalanine (pCPA) [serotonin (5-HT) synthesis inhibitor, 150 mg/kg] on the antidepressant potential of <i>Heracleum persicum</i> essential oil (HPEO) in the forced swim test (FST). Results are presented as mean ± SEM (n = 6). + p < 0.05 versus the HPEO group (500 mg/kg). *** P < 0.001 versus the vehicle group. Pretreatment with WAY100135 (5-HT1A/1B receptor antagonist, 10 mg/kg), ketanserin (5-HT2A serotonergic receptor antagonist, 5 mg/kg), and p-chlorophenylalanine (pCPA) [serotonin (5-HT) synthesis inhibitor, 150 mg/kg] on the antidepressant potential of <i>Heracleum persicum</i> essential oil (HPEO) in the forced swim test (FST). Results are presented as mean ± SEM (n = 6). + p < 0.05 versus the HPEO group (500 mg/kg). *** P < 0.001 versus the vehicle group.](https://services.brieflands.com/cdn/serve/3170b/a9c38baaecbf7a4d3ce0cc82dec186cb86ddd2a9/jrps-161974-i004-F4-preview.webp)
![Effects of <i>Heracleum persicum</i> essential oil (HPEO) on the serum antioxidant enzyme levels [glutathione (GSH), catalase (CAT) and nitric oxide (NO)]. The data are presented as mean ± SEM (n = 6). * P < 0.05, ** P < 0.01 and *** P < 0.001 versus the vehicle group; # P < 0.05 and ## P < 0.01 versus the fluoxetine (Flx) group Effects of <i>Heracleum persicum</i> essential oil (HPEO) on the serum antioxidant enzyme levels [glutathione (GSH), catalase (CAT) and nitric oxide (NO)]. The data are presented as mean ± SEM (n = 6). * P < 0.05, ** P < 0.01 and *** P < 0.001 versus the vehicle group; # P < 0.05 and ## P < 0.01 versus the fluoxetine (Flx) group](https://services.brieflands.com/cdn/serve/3170b/8164b239548ae6a26c63ffe5f7bbdba4e5dfbf4b/jrps-161974-i006-F6-preview.webp)