1. Background
Nowadays, the indiscriminate use of antibiotics and their subsequent introduction into the environment has raised global concerns, with an estimated 100,000 - 200,000 tons of antibiotics used annually worldwide (1). After the administration of these drugs, digestion and metabolism in the body lead to the excretion of residual compounds and their metabolites through human urine and feces (2). The presence of antibiotics has been detected in surface water, groundwater, wastewater, and even drinking water, in concentrations ranging from nanograms to micrograms per liter. These substances enter the environment through various sources, including sewage systems, pharmaceutical industries, hospitals, and human and animal waste (3). In recent years, pharmaceutical substances have been continuously discharged into the environment without restrictions. Although their entry rate into aquatic ecosystems may be low, their persistent release and cumulative effects pose a potential risk to aquatic organisms and microorganisms, warranting further consideration (4).
Among the various types of antibiotics, tetracyclines are the second most commonly produced and consumed group worldwide. These compounds are obtained naturally through the fermentation of certain fungi or synthesized via semi-synthetic processes, and they are frequently and indiscriminately used today to treat a wide range of infectious diseases (5, 6). Furthermore, the presence of these pharmaceuticals in the environment contributes to the development of antibiotic-resistant pathogens, which potentially threaten ecosystem stability and human health (4, 5). Conventional water and wastewater treatment methods are ineffective in breaking down and removing these compounds (3). Various strategies, including adsorption with activated carbon, reverse osmosis, air stripping, and biological treatments, have been employed to eliminate pharmaceutical contaminants. However, these methods merely transfer pollutants from one phase to another rather than fully degrade them.
In contrast, the use of HKUST@Agarose composites presents a promising approach for the decomposition of these compounds. The remarkable physical and chemical properties of metal-organic frameworks (MOFs) have drawn significant interest for diverse applications as composite materials. These highly ordered porous structures are formed via the self-arranging of metal ions and organic ligands (4, 5). Recently, polysaccharide-based MOFs have emerged as nanostructures with well-defined functions. Common dispersion methods for extractive adsorbents include vortex mixing, ultrasound, microwaves, and mechanical stirring (6). However, these techniques are impractical for on-site applications due to their reliance on external equipment. Boiling is a simple yet effective reaction that generates carbon dioxide, facilitating the complete dispersion of extracted solvents or adsorbents without additional energy, making it a viable method for in-field extraction (7).
Agarose is a polygalactan that is separated from agaria and directly from red seaweed purely. It offers high porosity, a hydrophilic structure, chemical stability, and relative neutrality, making it a valuable material for solid-phase extraction (SPE) and affinity chromatography (8). Agarose has been used to create homogeneous spherical particles, high-porosity beads, and porous films (9). However, membranes and particles made from agarose generally lack mechanical strength and stability (10). To enhance these properties, cross-links are introduced, forming bonds between hydroxyl groups in polysaccharide chains. Polysaccharide gels such as agar and agarose become porous at specific temperatures, with cavity sizes dependent on the concentration of polysaccharides. These hydrogen-bonded polysaccharide chains form well-structured porous materials (11). This class of MOFs provides enhanced biological adaptability and flexibility in hybrid materials due to their natural polysaccharide composition (12). In addition to exhibiting desirable biological properties such as biocompatibility and biosafety, the MOF-based polysaccharide composite studied demonstrates structural flexibility, excellent porosity, and chemical-thermal stability.
2. Objectives
Due to the lack of efficient and adequate methods for the removal of tetracycline from aqueous solutions, the present research project evaluates the effectiveness of agarose-coated metal-organic framework nanocomposite (HKUST&Agarose) as an auxiliary and complementary method in addition to some previously studied methods for the effective removal of this drug from natural sources. However, previous studies with other adsorbents such as activated carbon and graphene oxide, which have larger detection limits, smaller surface areas, and longer equilibration times, have shown poorer performance in competition with HKUST&Agarose.
3. Methods
3.1. Materials
In this study, we used HKUST and agarose polysaccharide to synthesize the agarose nanocomposite and also used sodium bicarbonate salt and citric acid to make effervescent tablets. Ethanol, methanol, acetonitrile, and acetone solvents were used as washing solvents, and tetracycline was used as an industrial model pollutant to create simulated wastewater. It should be noted that all the raw materials used are made by the German company Merck and Sigma-Aldrich. Here, by dissolving the appropriate amount of tetracycline in methanol, the standard main working solution is prepared, and then the working solutions are prepared by diluting the main working solution to a certain amount. HCl and NaOH with a concentration of 2-M were used to adjust the pH.
3.2. Devices
The X-ray diffraction (XRD) device model 6100 (STADIP) was used to determine the crystal structure of absorbent tablets. A UV-vis spectrophotometer, model PC 1650, manufactured by Shimadzu, Japan, was used for spectroscopic absorption measurement of tetracycline. A Fourier transform infrared photometer model FT-FR S 8500, Shimadzu, was used to check the IR spectrum of the material with KBr tablets, and the scanning electron microscopy model (Mira3mu) made by TE-SCAN was used to investigate the structure of the surface morphology and to investigate the constituent elements and how they are dispersed. The pH meter of HANNA model (microprocessor meter 211p) has been used to measure the pH. Also, to stir the solutions, the Bon-Marie shaker machine (Germany, Memmert WB14) was used.
3.3. Production of Agarose Nanocomposite HKUST
First, dissolve 0.4 g of agarose in 25 mL of water on a heater. Once the solution becomes clear and cools down, add 0.1 g of HKUST, which was synthesized according to previous reports (13), at room temperature. Stir the solution until it becomes uniform. Next, pour this mixture into a laboratory tube and allow it to cool. Once solidified, remove the agarose-HKUST composite from the tube and cut it into small tablets. The tablets are then placed in a freeze-dryer for 24 hours before being used as an absorbent for the extraction and measurement of tetracycline. To prepare effervescent tablets, citric acid and sodium bicarbonate are first completely dried in an oven. Then, 0.21 g of citric acid and 0.28 g of sodium bicarbonate are mixed thoroughly and compressed into tablets.
3.4. Device Method
In order to investigate the formed phases and crystal structure of the manufactured organic-metallic structure composite, XRD was performed using a Philips XRD instrument equipped with a copper (Cu-Kα) radiation source. The identification of phases and the determination of composite block sizes were carried out using the Xpert software, while the peak matching and comparison with the standard diffraction pattern (JCPDS) facilitated phase identification. The morphology, external geometry, phase differences, and structural variations of the metal-organic composite were examined using a field-emission scanning electron microscope (FE-SEM), specifically the MiRA3 model manufactured by TESCAN.
3.5. Procedure
To examine the absorption process of tetracycline, we add a tablet of adsorbent and a tablet of sodium bicarbonate and citric acid to 10 mL of solution containing tetracycline 5 ppm. After about 10 minutes, we take out the adsorbent tablet and wash it with a methanol solvent, and with the UV spectrometer device, the absorption is measured. Finally, the amount of tetracycline absorbed by the adsorbent, Qe, was calculated with Equation 1.
where C0, Ce are the initial concentration of the solution and the concentration of the solution in time t, V is the volume solution in liters (L), m the adsorbent value (g) and Qe are the adsorption capacity of the composite of the organo-metallic structure in terms of (mg/g). So the percentage of tetracycline was obtained using Equation 2.
4. Results
4.1. Microstructural Analysis
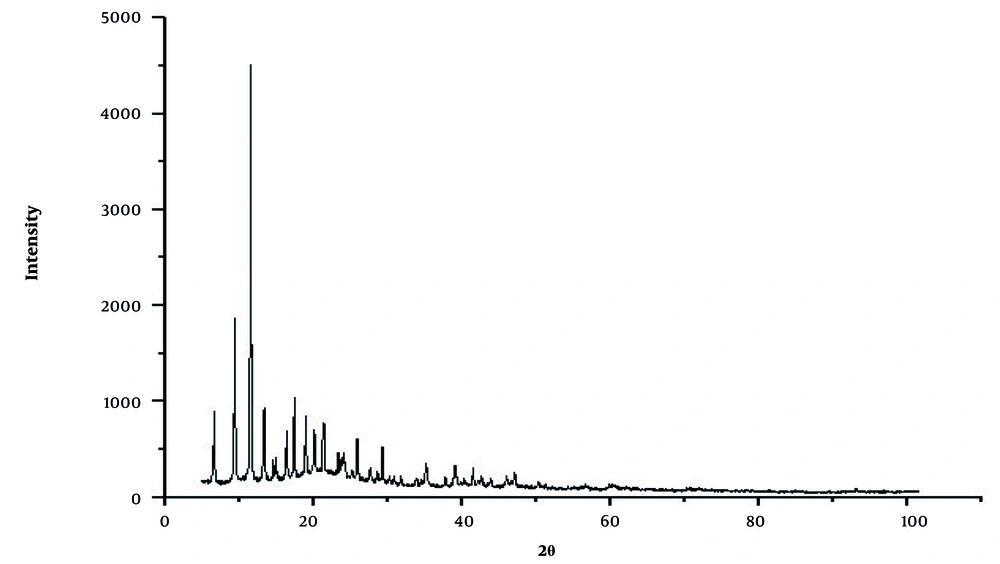
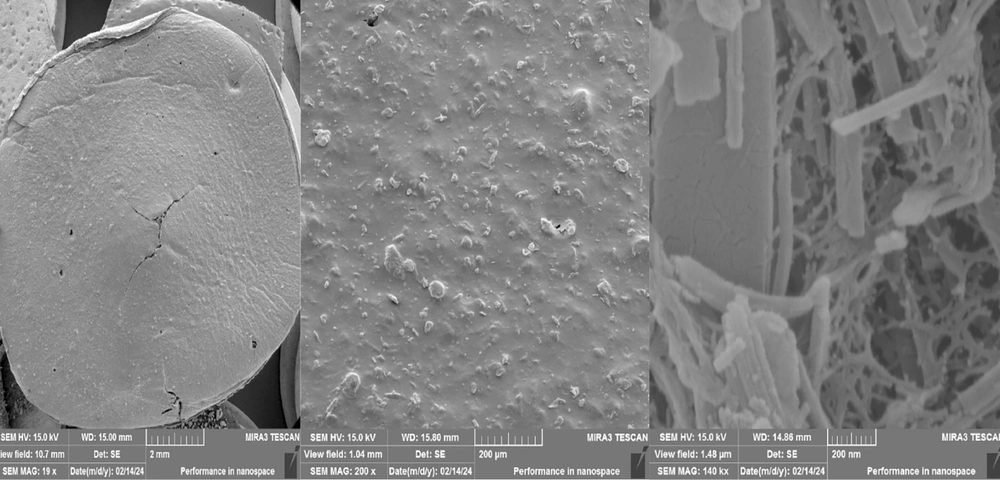
X-ray diffraction analysis was used to investigate the formed phase, measure the distance between crystal plates, measure the average size of crystals, and identify the structure of the material for the synthesized sample. Figure 1 shows the XRD pattern of the composite organometallic structure. The XRD pattern, the average block size, and the average distance between the crystal plates of the synthesized organic metal structure composite are presented. Additionally, the image taken from the scanning electron microscope (SEM) is shown in Figure 2. The conditions of this study are completely dry, and the synthesized metal-organic composite exhibits a rod-like morphology. Similarly, SEM images in Figure 2 clearly show the tubular structure of the nanocomposite of HKUST&Agarose.
4.2. Choosing the Type and Volume of the Elution Solvent
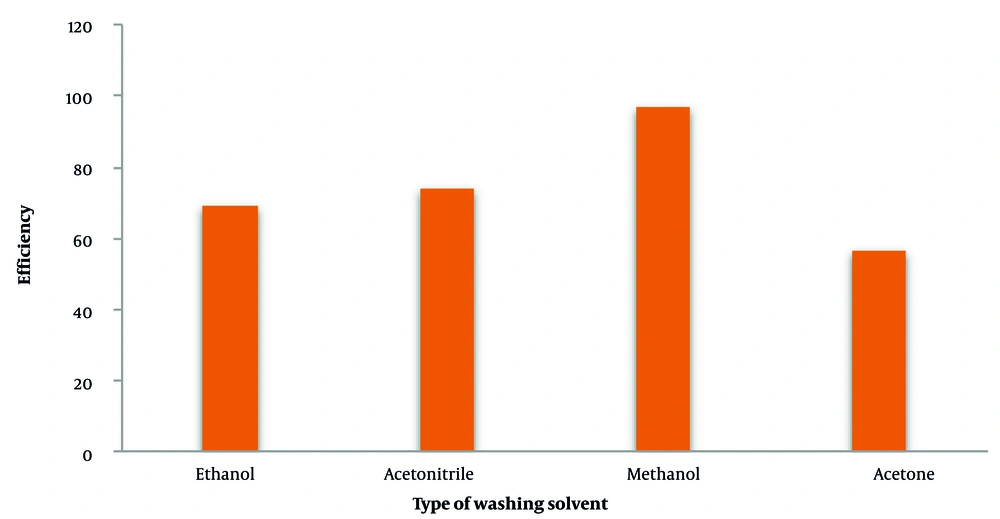
To remove tetracycline from the adsorbent surface without damaging it, the washing solvent must have this ability. In this regard, ethanol, acetonitrile, methanol, and acetone solvents were selected for this purpose, and tests were performed using each of these solvents as a washing solvent. Figure 3 compares the results of tetracycline washing with different solvents. Based on the results, methanol was selected as the washing solvent.
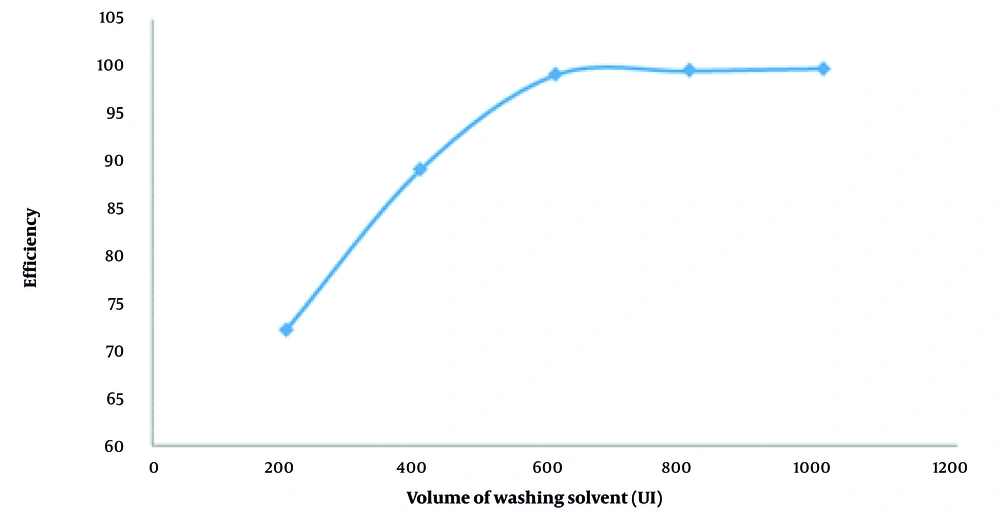
The amount of washing solvent in the aforementioned extraction method is directly related to the amount of adsorbent. The volume of the washing solvent should be sufficient to wash all the tetracycline from the adsorbent surface but not so high as to reduce the preconcentration factor. Different amounts of methanol (200, 400, 600, 800 μL, etc.) were studied, and based on the results presented in Figure 4, a volume less than 600 μL is insufficient for the complete washing of the adsorbent from tetracycline, with a significant decrease in extraction efficiency observed at 400 μL. After a volume of 600 μL, further increases in solvent volume have no effect on the extraction efficiency, as all tetracycline is separated from the adsorbent surface. Therefore, 600 μL of methanol was selected as the optimal amount and was used in the subsequent stages.
4.3. Optimization of Effective Parameters for Tetracycline Extraction
The multivariate optimization method is one of the methods used in optimization. Unlike the univariate method, this approach emphasizes the interactions between factors simultaneously, allowing for quicker and more efficient results. It also provides the possibility of optimizing several variables at the same time. The design of the experiment with the multivariate optimization method consists of three parts: (1) First, the factors affecting the testing and analysis process are introduced; (2) then, the experiments are designed; (3) finally, statistical analysis is performed.
The surface response methodology can investigate the interaction between different factors, and statistical analysis is performed with the graphs provided by this method. In this method, three primary factors were introduced, each evaluated at two levels, and the experimental design was carried out using Minitab 17, a statistical modeling software, within the framework of design of experiments (DOE). The pH of the analyte solution, contact time, and ionic strength effect were the three variables available in the design. The high and low levels for each variable are given in Table 1, according to the results of some preliminary experiments.
| Parameters | Factors’ Levels | |
|---|---|---|
| Low | High | |
| pH | 3.5 | 6 |
| Effect of time (min) | 1 | 10 |
| Salt addition (%) | 2 | 6 |
The Factors Included in the Central Composite Design and the Levels for Each of Them
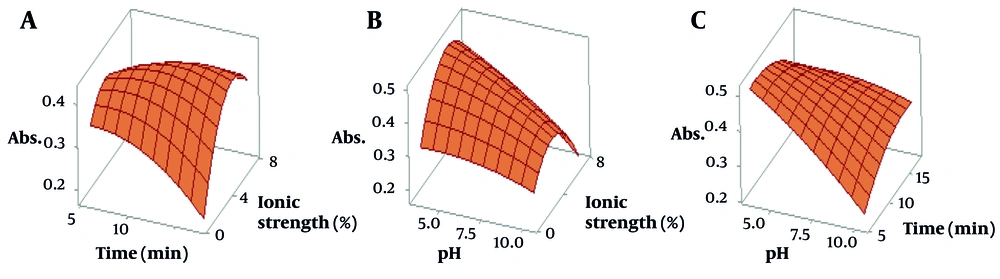
Figure 5 shows the 3D color scheme of the surface response image for the preceding main interaction. This design is useful for describing the response as a function of each pair of graphically independent parameters. The optimal values can be selected by visual examination and based on the highest desirability. The best response was obtained under the analysis solution with pH = 4.0, time = 5.5, and ionic strength = 4.
The three-dimensional representation illustrates the significance of key parameters in optimizing adsorption efficiency. Specifically, it highlights A, the interaction between duration (time) and ionic strength; B, the relationship between ionic strength and pH; and C, the effect of time and pH on attaining optimal conditions with the highest desirability. These factors collectively contribute to maximizing the adsorbent’s performance in the adsorption process.
In Figure 5A of the simultaneous study, ionic strength-contact time is observed. By increasing the time up to 5.5 minutes and increasing the ionic strength up to 4%, the removal has increased, and then it shows a constant trend. In Figure 5B, the simultaneous effect of two parameters, ionic strength and pH, is shown, and in Figure 5C, the simultaneous effect of contact time and pH parameters is shown. According to the results seen in Figure 5B and C, in neutral and acidic pH, the absorption value is at the desired level, and in the basic pH, its desirability degree is reduced. In acidic pH, due to the cationic form of the nanocomposite, electrostatic interactions can be somewhat effective in increasing the removal rate (14). Therefore, pH = 4.0 was selected for further studies.
Although a precise understanding of the adsorption mechanisms in such studies remains challenging, the authors propose that, in addition to the electrostatic interactions described, hydrogen bonding may occur between the analyte and the adsorbent. This is likely due to the presence of hydroxyl groups in the tetracycline structure and agarose, as well as the oxygen and nitrogen atoms in the HKUST framework. Furthermore, the presence of aromatic rings in both the drug and the adsorbent may facilitate π–π stacking interactions. Collectively, these factors could account for the adsorption mechanism of the drug on the nanocomposite surface.
4.4. Calibration Curve
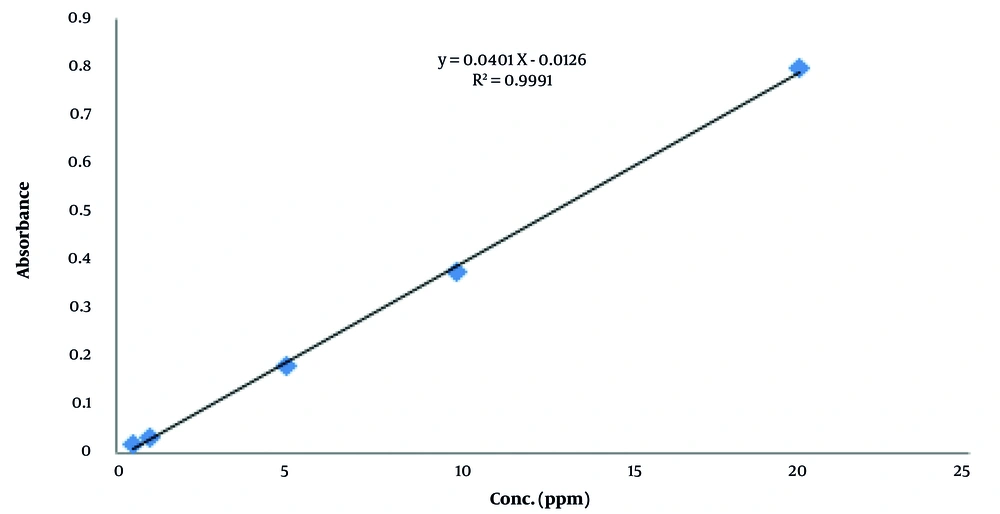
To draw the calibration curve and obtain the linear range, appropriate concentrations of tetracycline were prepared with appropriate dilution from the mother solution under optimal conditions and injected into the UV device (Figure 6). Under optimal conditions, tetracycline exhibits linear behavior within the concentration range of 0.09-20 mg/L. The standard deviation of the blank measurement was 4.2 × 10-4, and the correlation coefficient was 0.9991. Some of the analytical characteristics of the method are summarized in Table 2.
| Characterization | Analytical Parameters |
|---|---|
| Linear equation | y = 0.0401x - 0.0126 a |
| Linear range (mg/L) | 0.09 - 20 |
| Concentration range (mol/L) | 4.5 × 10 - 5 - 2 × 10 - 7 |
| Determination coefficient | 0.9991 |
| LOD (mg/L) | 0.032 |
| EF b | 16.7 |
Analytical Parameters of the Proposed Method for Measuring Low Levels of Tc
The reproducibility of the method was investigated by measuring the standard tetracycline sample, 5 times under optimal conditions (adsorbent value, washing solvent volume, pH 4.0, extraction time 5 minutes and salt concentration of 4%). In this condition, the efficiency 96.6% and the relative standard deviation 3.68 were calculated for the measurement of tetracycline after separation and absorption measurement. The detection limit of the method was calculated to be 0.032 mg/L based on the equation (LOD = 3Sb/m). Where LOD is the detection limit, Sb is the standard deviation for 10 times of control measurement and m is the slope of the calibration curve (15).
4.5. Determination of Tetracycline in Real Samples
To determine whether the adsorbent can be applied to real samples, the method was used to determine tetracycline in five different serum samples. Acetonitrile was used to deproteinize the serum. First, add 1 mL of acetonitrile to the serum and allow 10 minutes for the deproteinization to complete, during which the proteins settle at the bottom of the container. To completely isolate the precipitated proteins, the solution is centrifuged for 10 minutes at 6,000 rpm. The spiked serum solution was placed under optimal conditions. All five samples did not initially contain tetracycline, so tetracycline was spiked at a concentration of 5 ppm. To extract and measure tetracycline by the mentioned method, 10 mL of the solution was taken and measured under optimal conditions. Table 3 shows the results of this work. For three replicated analyses, relative standard deviations of 3.9 to 5.6% were obtained for the studied samples. Similarly, it seems that the nanocomposite showed good performance in real environmental samples, with the highest recovery rate (99.6%) in serum samples and the lowest corresponding to wastewater discharge points with a recovery of 87%. This is probably due to complex matrix effects from organic matter and heavy metals, highlighting the need for pretreatment in highly polluted environments.
| Real Sample | Before Spike (ppm) | After Spike (ppm) | Efficiency (%) | RSD (%) |
|---|---|---|---|---|
| Serum sample 1 | - | 4.98 | 99.6 | 4.2 |
| Serum sample 2 | - | 4.83 | 96.6 | 3.9 |
| River (upstream) | - | 4.77 | 95.4 | 4.3 |
| River (sewage discharge) | - | 4.35 | 87.0 | 5.6 |
Analytical Characteristics of the Proposed Method for Determining Tetracycline
4.6. Challenges and Limitations in Tetracycline Separation and Quantification
The structural resemblance between tetracycline and its analogs, including minocycline and doxycycline, can lead to complications in accurately measuring tetracycline concentrations. This similarity may introduce analytical errors and reduce measurement precision. However, the simultaneous presence of all these compounds in a single sample is unlikely. Employing sophisticated analytical methods, such as high-performance liquid chromatography (HPLC), can help minimize these interferences, though it does not entirely resolve the issue. In blood serum samples, proteins can influence the adsorption and separation behavior of tetracycline. This effect is particularly relevant when attempting to isolate tetracycline at low concentrations, as it may pose additional difficulties in achieving precise quantification. To expand the scope of this study, the same extraction approach could be tested on other antibiotics, such as ampicillin and ciprofloxacin, to assess whether the HKUST@Agarose nanocomposite demonstrates comparable effectiveness. Consequently, future research should emphasize the design and optimization of advanced nanocomposites with improved selectivity and stability to enhance analytical performance.
4.7. Evaluation of the Proposed Method with Other Methods
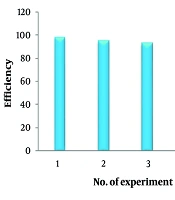
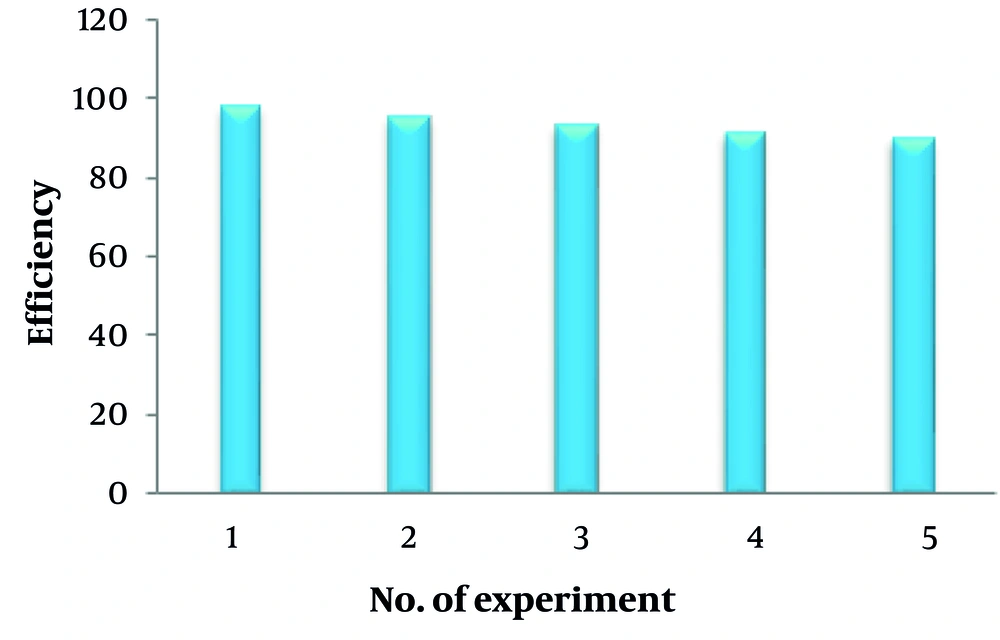
Table 4 provides a comparative overview of the analytical features of the proposed method and other recently developed automated techniques for tetracycline determination across various sample types. The findings indicate that the proposed method achieves comparable performance in terms of LOD, linear range, and accuracy to approaches utilizing extraction combined with HPLC-UV analysis. In addition, HKUST@Agarose nanocomposites, due to their magnetic properties, can serve as suitable recyclable adsorbents for up to five cycles, retaining 90% efficiency at the optimal methanol concentration of 70 - 80% for tetracycline desorption, with a contact time of five minutes (Figure 7). It is also evident that SPE-based methods offer enhanced sensitivity, but this advantage is accompanied by high sample consumption and lower sampling frequencies. While the proposed technique for tetracycline detection is both simple and efficient, it faces a limitation in its inability to distinguish individual compounds without preliminary separation. Despite this, the proposed method eliminates the need for costly instrumentation, such as chromatographs or fluorometers, which are integral to other techniques. Furthermore, it offers an environmentally friendly alternative, minimizing the disposal of mobile-phase solvents, a significant concern associated with liquid chromatography methods.
| Methods | Removal Percentage (%) | Type of Sample | LOD (μg/L) | Linear Range (μg/L) | RSD (%) | Sampling Frequency (min-1) | Ref. |
|---|---|---|---|---|---|---|---|
| Spectrophotometry | 98.04 - 101.63 | Pharmaceuticals | 400 | 1000 - 40000 | 0.2 - 1.5 | 52 | (16) |
| MSPE-spectrophotometry | 91 - 97 | Milk | 10 | 30 - 600 | 1.6 -4.3 | 2 | (17) |
| SALLME-HPLC-UV | 91 - 105 | Urine | 170 | 500 - 20000 | 4.0 - 6.0 | 9 | (18) |
| SPE-HPLC-UV | 68.3 - 97.9 | Honey | 8 | 50 - 1000 | 4.1 - 7.1 | 4 | (19) |
| Spectrophotometry | 99 | Honey | 130 | 400 - 25000 | 1.0 - 5.0 | 60 | (20) |
| SPE-fluorometry | 87 | Wastewater | 9 | 30 - 1000 | 0.5 - 3.9 | 3 | (21) |
| Nanocomposite adsorbent | 99.6 | Serum | 32 | 90 - 20000 | 3.68 | 5.5 | The present study |
Analytical Characteristics of the Proposed Method Compared to Previously Reported Automated Methods for Determining Tetracycline
5. Discussion
To investigate the formed phases and crystal structure of the produced organic-metallic structure composite, XRD was performed using a Philips XRD instrument equipped with a copper (Cu-Kα) radiation source. The identification of phases and the determination of composite block sizes were carried out using the Xpert software, while peak matching and comparison with the standard diffraction pattern (JCPDS) facilitated phase identification. The morphology, external geometry, phase differences, and structural variations of the metal-organic composite were examined using a FE-SEM, specifically the MiRA3 model manufactured by TESCAN.
In summary, this study reports the creation and characterization of a bio-metallic framework nanocomposite (HKUST@Agarose) prepared via an in situ self-arranging method, a green synthesis approach. Following its identification, the synthesized organometallic nanocomposite was applied to remove tetracycline, an environmental pollutant. The structure of the nanocomposite was characterized using scanning electron microscopy and XRD techniques. To optimize tetracycline removal, the effects of pH, ionic strength, adsorbent dosage, contact time, as well as the type and volume of the elution solvent were systematically studied.
5.1. Conclusions
According to the results of this study, an agarose-coated structure of metal-organic nanocomposite was synthesized using a simple, low-cost, low-risk method, and also with reduced pollution from the synthesis using biocompatible materials. In addition, the synthesized nanocomposite is regenerable. It is noteworthy that the experimental results showed that under acidic conditions, with a contact time of 5.5 minutes and an adsorbent dose of 300 mg, the nanocomposite achieved the maximum adsorption capacity of tetracycline.