1. Background
Hypertension is a major health problem that significantly increases the risk of several health problems, such as cardiovascular disease (CVDs), coronary heart disease (CHD), congestive heart failure (CHF), ischemic and hemorrhagic stroke, kidney failure, and peripheral vascular disease. Although antihypertensive therapy significantly reduces the risk of CVDs and renal problems, most people with hypertension do not receive treatment at all, or their treatment is inadequate (1). There are different levels of hypertension and age-related increase in blood pressure. In industrial societies, blood pressure increases steadily during the first two decades of life (2).
Recent studies have shown that adipose tissue hormones or adipokines are involved in the pathogenesis of obesity complications such as hyperlipidemia, diabetes mellitus, hypertension, atherosclerosis, and heart failure. Apelin, as an Adipokine, exists in the body in at least three forms of 13, 17, and 36 amino acids, all of which originate from a 77 amino acid precursor. Apelin is a multifunctional peptide involved in the regulation of the cardiovascular system (including regulation of blood pressure and cardiovascular function control). So obviously, Apelin has a role in the pathophysiology of hypertension and heart diseases associated with high blood pressure (3, 4).
It also plays a role in angiogenesis and apoptosis of endothelial and smooth muscle cells. Besides, it has an anti-vascular endothelial growth factor. In blood flow, Apelin modifies the expression of the endothelial nitric oxide synthase enzyme (eNOS) and causes vasodilation related to eNOS, and acts against the vasoconstriction associated with angiotensin II. Therefore, it has positive inotropic and protective effects on the heart. Apelin is a protein that affects the vascular endothelium-dependent vasodilation and with positive inotrope. Apelin not only can reduce the ventricular afterload and preload, but also can increase the strength of cardiac contraction. Apelin plasma level is a practical guide for evaluating the severity of heart failure (3, 4).
This peptide acts through Apelin’s receptor (APJ receptor) and is much similar to angiotensin II. Apelin is widely spread in many tissues, mostly vascular endothelium (5). Apelin/APJ System has several physiological effects on water/electrolyte balance, blood sugar control, and safety and nutritional status of the body, but its main purpose is the cardiovascular system (6). Some studies showed that the apelin/APJ system has an essential role in body normal function as well as CVDs such as atherosclerosis, CHD, heart failure, and systemic/pulmonary hypertension (7).
Apelin generally creates autocrine and paracrine effects, but its plasma level also has physiological effects. Apelin plasma concentration is around 10 g/mL, and its half-life is less than 5 minutes. The slow, stable, and inotropic effects of apelin have also been proved in very low concentrations up to the point that Apelin has been known as the strongest inotrope endogenous molecule, even more, effective than adrenomedullin and endothelin (6, 8, 9).
On the other hand, the mutual effect of Apelin-APJ receptor on vein surface causes vascular vasodilation induction through NO release from endothelial cells and declines vascular load in the left ventricle and its filling pattern improvement through decreasing the systemic vascular resistance. APJ receptor exists in the smooth veins and causes its plasma level maintenance (10, 11).
2. Objectives
Since Apelin plays an important role in blood pressure regulation, the plasma Apelin level in hypertensive patients receiving hypertensive drugs has been measured in this study in order to inform the hypertensive patients about the importance of this peptide as well as preventing the usage of its devastating materials.
3. Methods
The study population of the present research was all hypertension patients admitted to the cardiology clinic of Vali-e-Asr Hospital of Birjand in 2014 with the arrival conditions. Participants were selected using a non-random sampling method. The inclusion criteria were being aged 25 to 85 years, satisfaction to participate, the absence of co-morbidities such as diabetes, dyslipidemia, CAD, hypertension diagnosis for patients in the recent two years, complete treatment period, regular referring to the cardiology clinics, and regular consumption of medications, lack of uncontrolled hypertension, absence of any secondary hypertension, chronic renal and hepatic diseases, diabetes, CAD, and history of, or current risk of cancer.
In total, 140 eligible subjects were divided into four groups: (A) 45 hypertensive patients who were treated with an Angiotensin II receptor blockers (ARB) drug such as losartan; (B) 31 hypertensive patients treated with calcium channel blockers (CCB) drugs such as amlodipine; (C) 33 hypertensive patients, simultaneously treated with an ARB drug such as losartan and a CCB drug such as amlodipine; and (D) 31 healthy subjects as controls. Demographic characteristics of participants were collected using questionnaires, and their blood samples were also collected.
All sampling procedures were performed by nurses under sterile conditions. Patients were ensured that their blood samples only will be used for research purposes, and their information would remain confidential. To take a blood sample, 5 cc of blood was taken from the patients referring to the cardiology clinics. After centrifugation, the blood serum samples were divided into 5 mL tubes and then frozen and stored at -20°C.
Collected samples were then analyzed within 2 to 4 weeks, and Apelin levels were evaluated using an ELISA kit (East Biopharm Company of Thailand). Blood pressure measurement was performed using a mercury manometer in a quiet environment on the cases’ right hands in a sitting position. The patients were asked not to use energetic substances, narcotics, tea, soft drinks, and cigarettes an hour before assessing their blood pressure, and they were also asked to rest on a chair for 5 minutes before the assessment. The study was approved by the research ethics committee of the Birjand University of Medical Sciences (ethic code: Ir.bums.1394.287). Besides, the principles of the Declaration of Helsinki and its amendment were followed.
Data were analyzed using SPSS (V.16) in order to determine the distribution of the main variable (Apelin). According to the results of the Kolmogorov-Smirnov test (P = 0.144), the non-normality assumption was rejected, and therefore the distribution was assumed to be normal. In addition to descriptive statistics (i.e., mean and standard deviation), data were analyzed using the statistical tests (t-independent, ANOVA, and Tukey tests). Statistical significance was considered when P-value < 0.05.
4. Results
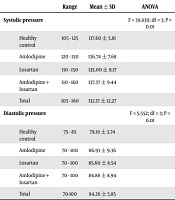
In the present study, 140 eligible subjects were evaluated, as follows: 31 subjects in the healthy group, 31 in the amlodipine group, 45 in the losartan group, and 33 in the combination therapy group (amlodipine + losartan). There was no significant difference between the groups concerning gender and mean age (P = 0.467 and P = 0.276, respectively) (Table 1). However, significant differences were observed among the different groups of patients compared to healthy controls concerning mean systolic blood pressure, according to ANOVA test results (P = 0.01). According to the results of the Tukey HSD test, the only significant difference was between the mean diastolic blood pressure of healthy people and other therapeutic groups, and in this regard, there was no difference among treatment groups (Table 2). The results of the ANOVA test for comparing the values of Apelin demonstrated a significant difference between the groups; therefore, the post hoc test was used to identify the precise difference.
| Group | Sexuality, No. (%) | Chi Square Test | Groups | Age | ANOVA | ||
|---|---|---|---|---|---|---|---|
| Male | Female | Range | Mean ± SD | ||||
| Healthy control | 13 (40) | 18 (60) | Value = 2.607; df = 3; P = 0.467 | Healthy control | 39 - 76 | 9.63 ± 52.56 | F = 1.303; df = 3; P = 0.276 |
| Amlodipine | 10 (32.3) | 21 (67.7) | Amlodipine | 39 - 76 | 9.56 ± 52.32 | ||
| Losartan | 22 (48.9) | 23 (51.1) | Losartan | 34 - 80 | 9.22 ± 53.33 | ||
| Amlodipine+ losartan | 16 (48.5) | 17 (51.5) | Amlodipine+ losartan | 44 - 73 | 7.74 ± 56.27 | ||
| Total | 60 (43.2) | 79 (56.8) | Total | 34 - 80 | 9.09 ± 53.64 | ||
Different Groups of Patients Based on Gender and Age
| Range | Mean ± SD | ANOVA | |
|---|---|---|---|
| Systolic pressure | F = 39.830; df = 3; P = 0.01 | ||
| Healthy control | 105 - 125 | 117.60 ± 5.81 | |
| Amlodipine | 120 - 150 | 136.74 ± 7.68 | |
| Losartan | 110 - 150 | 135.00 ± 9.17 | |
| Amlodipine + losartan | 110 - 160 | 137.37 ± 9.44 | |
| Total | 105 - 160 | 132.17 ± 12.27 | |
| Diastolic pressure | F = 5.552; df = 3; P = 0.01 | ||
| Healthy control | 75 - 85 | 79.19 ± 3.74 | |
| Amlodipine | 70 - 100 | 86.93 ± 9.36 | |
| Losartan | 70 - 100 | 85.60 ± 8.54 | |
| Amlodipine + losartan | 70 - 100 | 84.88 ± 8.94 | |
| Total | 70-100 | 84.26 ± 5.85 |
Different Groups of Patients Based on Systolic and Diastolic Blood Pressure
The results of the Tukey’s test indicated that the highest level of Apelin existed in order in the amlodipine + losartan, losartan, and amlodipine treatment groups, respectively. Meanwhile, Apelin level in patients receiving losartan and the combination of losartan and amlodipine was significantly higher compared to the amlodipine-treated group (P ≤ 0.05). Compared to the healthy individuals, the groups treated with amlodipine, losartan, and combined therapy had lower circulating levels of apelin, respectively (Table 3). In the present study, most of the participants (119 patients, 85%) had a low level of Apelin (less ≥ 365 pg/mL). Assessing the quality level of Apelin among different groups showed a significant correlation (P = 0.01) (Table 4).
| Group | Range | Mean ± SD | ANOVA |
|---|---|---|---|
| Apelin, pg/mL | F = 55.308; df = 3; P = 0.01 | ||
| Healthy control | 290 - 415 | 366.16 ± 36.04 | |
| Amlodipine | 199 - 301 | 247.19 ± 27.77 | |
| Losartan | 198 - 375 | 282.93 ± 47.08 | |
| Amlodipine + losartan | 210 - 335 | 289.84 ± 32.20 | |
| Total | 198 - 415 | 295.07 ± 55.49 |
Different Groups of Patients Based on the Serum Apelin Level
| Group | Normal, No. (%) | Low, No. (%) | Exacts Fisher Test |
|---|---|---|---|
| Apelin, pg/mL | Value = 47.656; P = 0.01 | ||
| Healthy control | 18 (58.1) | 13 (41.9) | |
| Amlodipine | 0 (0) | 31 (100) | |
| Losartan | 3 (6.7) | 42 (93.3) | |
| Amlodipine + losartan | 0 (0) | 33 (100) | |
| Total | 21 (15) | 119 (85) |
Different Groups of Patients Based on the Quality Level of Serum Apelin
The results depicted a significant association between the mean systolic and diastolic blood pressure levels and the level of Apelin; i.e. those with a low Apelin level had much higher mean systolic and diastolic blood pressures compared to those with normal Apelin level (P < 0.01 and P < 0.05, respectively) (Table 5).
| Apelin | Mean ± SD | Independent t-test |
|---|---|---|
| Systolic blood pressure | t = 8.63; df = 137; P = 0.01 | |
| Low level | 143.91 ± 9.56 | |
| Normal | 115.90 ± 5.46 | |
| Diastolic blood pressure | t = 3.14; df = 137; P = 0.02 | |
| Low level | 85.16 ± 8.68 | |
| Normal | 78.90 ± 4.75 |
Average Level of Systolic and Diastolic Blood Pressure in Subject with Normal and Low Level of Apelin
5. Discussion
Losartan is an antihypertensive drug acting as a selective angiotensin II type 1 (AT1) receptor antagonist (12). In addition, Amlodipine is a CCBs drug that exerts its action through inhibition of calcium influx into vascular smooth muscle cells and myocardial cells. Indeed, Amlodipine decreases peripheral vascular resistance (PVR), which is indicated for treating high blood pressure (13).
The current study aimed to compare the serum Apelin levels in high blood pressure patients treated with amlodipine, losartan, and amlodipine + losartan. The highest Apelin level was observed in patients treated with amlodipine + losartan, which was significantly higher than those who received losartan and amlodipine alone. The systolic blood pressure was higher in the amlodipine + losartan group compared to the amlodipine and losartan groups, but this difference was not significant. Furthermore, the diastolic blood pressure level was higher in amlodipine group compared to the losartan and amlodipine + losartan groups; however, it was not statistically significant. Those in the control group had a lower systolic and diastolic blood pressure and higher levels of Apelin compared to the treatment groups. Regarding the higher blood Apelin level in groups treated with losartan or losartan + amlodipine, compared to the group treated solely with amlodipine, we can mention the results of Hung et al. (14), which attempted to analyze the performance of Ang II receptor during Adipocytes differentiations. They concluded that inhibiting the renin-angiotensin-aldosterone system causes increased secretion of Apelin; a conclusion that is in line with the results of the present study (14). Furthermore, Siddiquee et al. (15), in a study on the protective effects of Apelin against the cardiovascular fibrosis resulting from angiotensin II and PAI-1 production decline, demonstrated that Apelin has protective properties against vascular remodeling and cardiac fibrosis through the direct regulation of PAI-1 gene expression. The mentioned protective effect is induced by the synergistic inhibition of Ang II signaling and NO production rise due to Apelin (15). The results obtained regarding the lower level of diastolic blood pressure in the group treated with losartan, as compared to treatment groups, can be justified in this respect.
Akcilar et al. (16) also showed that apelin decreases blood pressure in DOCA-salt rats and can be used as a therapeutic agent in the treatment of high blood pressure in the future. The result obtained in our study is in line with this study (16).
It is noteworthy that Apelin is not only effective in systemic blood pressure, but it also plays an important role in regulating pulmonary hypertension, as reported by Wannamethee et al. (17) and Azizi et al. (18). According to the literature, there is a predictive effect of Apelin polymorphism in patients with hypertension treated with losartan, in women unlike men, that means the observed decrease in systolic blood pressure after 24 weeks of treatment with losartan was significantly different in dominant and recessive genotype models. Therefore, it has been concluded that there is a relationship between the existent of specific Apelin genotype and a better response to treatment with Angiotensin II inhibitor (losartan) (19).
In line with the results of this study regarding the higher Apelin levels in healthy people with normal blood pressure, Przewlocka-Kosmala et al. (20) demonstrated that the Apelin level decreased in blood circulation in the patients with high blood pressure and the low plasma apelin level in this patients can independently aggravate the ventricular systolic and diastolic functional disorder.
Andersen et al. (21) investigated the association between the Apelin level and pulmonary hypertension and showed that patients with primary pulmonary hypertension (PPH) had a lower level of plasma and decreased expression of Apelin in lung endothelial cells. So Apelin has been presented as a potential marker for PPH. In addition, Apelin plays a role in angiogenesis and regulating the apoptosis of endothelial, smooth muscle cells. Chronic treatment with Apelin could reduce the pulmonary hypertension progression in animal models, and researchers have suggested APLNR as an interesting potential therapeutic target for PPH (22-24).
Some studies have investigated the effect of Apelin on blood pressure. For example, Fan et al. (25) investigated the role of Apelin in the prevention of pulmonary hypertension induced by hypoxia in rats. They reported that Apelin showed an important role in the treatment of hypoxic pulmonary hypertension of rats based on vasodilation of pulmonary artery and inhibition of oxidative stress (25).
This study demonstrated that Apelin has a protective effect in the prevention of hypertension in healthy subjects, and for those who suffer from hypertension, it can decline the level of Apelin. Also, according to the findings, renin-angiotensin-aldosterone system inhibitor was associated with an increased level of Apelin, which translates into better response to treatment.
Considering the effects of Apelin gene polymorphism (additive, dominant, and recessive genotypes) on the response rate of patients suffering from high blood pressure to antihypertensive therapies, the researchers suggest evaluating this association in future studies. More comprehensive studies with larger sample sizes that contain other high blood pressure groups such as malignant hypertension are suggested. Furthermore, considering the effect of Apelin level in more optimal control of blood pressure and improving the response to antihypertensive treatments, clinical trial studies are highly recommended.