1. Background
COVID-19 is a very contagious disease caused by SARS-CoV-2 (1, 2). COVID-19 presents symptoms including fever, dry cough, fatigue, headache, joint pain, asymptomatic or flu-like symptoms causing loss of taste and smell, pneumonia, and acute respiratory distress syndrome (3-5). Various vaccine models are made against COVID-19 by many government and private institutions around the world using many different methods. All vaccine trials developed are examined in detail by the World Health Organization, and those that meet the minimum requirements are approved and released to the market (6).
Vaccine studies should be emphasized during pandemics because healthy people should be protected from disease, epidemics should be prevented, and deaths should be stopped. However, all these processes do not progress as quickly as in practice. Until vaccines with adequate safety and efficacy are produced, epidemics get out of control and become pandemics. At this stage, hospitalizations increase, the number of deaths accelerates in parallel, and the maximum patient capacity of the hospital to provide care is exceeded. For this reason, all vaccines whose production is completed are subject to review by the World Health Organization, and those that meet the minimum requirements are approved for immediate use and distributed to the market.
Some of the vaccines that were decided to be used urgently in the COVID-19 pandemic in the world are also used in Turkey. Sinovac (CoronaVac), BNT162b2 (Pfizer-BioNTech), and Turkovac vaccines are still administered in Turkey (7). The Pfizer-BioNTech vaccine is the first mRNA-based vaccine urgently approved during COVID-19. Various side effects may occur after the entire vaccination administration. These side effects were generally determined as fatigue, muscle discomfort, itching, fever, edema, tingling, joint pain, headache, and chills (8). However, it is still impossible to predict all the effects of Pfizer-BioNTech's vaccine, produced with a new technique whose long-term effects are not fully known (9). CoronaVac is the first inactivated virus vaccine produced in the early days of the pandemic and put into practice against COVID-19. With the announcement of phase I/II trial results, it has been approved for emergency use in some countries. In later studies, it was stated that the devastating side effects of the CoronaVac vaccine were not recorded in Phases I-II-III, and all the side effects observed were tolerable (10). The vaccine named Turkovac, developed by the Presidency of Health Institutes of Turkey (TÜSEB) started a phase 1. Phase trial in 2020 and was approved for emergency use at the end of 2021; Turkovac was included in the list of uses and started to be applied (11).
All vaccines developed create hope in humans but also create fear. Young adults, in particular, question vaccinations. In addition, especially university students in Turkey were required to obtain HES code approval at the entrance to the building in order to attend their classes (Hayat Eve Sığar (HES): A digital system where the Ministry of Health inquires online whether the person has COVID-19, contact with COVID-19 or has been vaccinated). A vaccination card with a digital data matrix indicated that the people were vaccinated. Negative PCR testing was mandatory for those who could not show the vaccination card. Negative PCR test results are mandatory for unvaccinated people to enter public institutions and businesses. For this reason, it is imperative to determine the ideas of young adults about the vaccine, rather than the elderly, and the signs and symptoms they experience after vaccination. There are not enough studies to determine the long-term effects of vaccines approved for emergency use, the level of trust and immunization with these vaccines, and individual opinions about vaccines.
2. Objectives
This research aimed to define the status of young university students in Turkey with the COVID-19 vaccine and their ideas about it.
3. Methods
This descriptive and cross-sectional study consisted of 6,254 undergraduate students studying at a state university in the Central Anatolia region. The study sample was created with a ±5% margin of error and a 95% confidence interval. It was determined that the study should be conducted with at least 384 students using the sample rate size formula (P = 0.5, 1- P or q = 0.5). The number of students enrolled in undergraduate faculties at the university was considered, and the number of samples was calculated using the stratification method. It was decided to admit 55 students from the Faculty of Medicine, 33 from the Faculty of Engineering and Architecture, 34 from the Faculty of Theology, 13 from the Faculty of Dentistry, 68 from the Faculty of Health Sciences, 35 from the Faculty of Education, 82 from the Faculty of Sport Sciences, and at least 64 from the Faculty of Administrative Sciences. The research was completed with a total of 602 students.
Undergraduate students enrolled in the university in the 2021 - 2022 academic year, volunteered to participate in the research, and answered all questions completely were included in the study.
The data were collected through face-to-face interviews using a questionnaire prepared by the researchers after conducting a literature review and getting expert opinions on the infection. The answers were expressed as a percentage. A questionnaire was applied randomly to the students. Each form took an average of 15 minutes to complete. The questionnaire consisted of nine questions to determine the sociodemographic characteristics (age, income, living area, etc.) and 23 questions (vaccination status, type of vaccine, the incidence of side effects, etc.) to determine their opinions about getting vaccinated. The only question was, “what is your general opinion about the vaccine.” The students were asked to mark one of these propositions: “I don’t know” “I think positively about the vaccine” or “I think negatively about the vaccine”.
Statistical package for the social sciences 21.0 (SPSS; IBM Corporation) program was used for statistical analysis. Frequency, standard deviation, mean, and minimum-maximum were examined for descriptive analysis. The chi-square test was used to determine the differences between groups. The statistical significance was accepted at P < 0.05.
Institutional permission and ethical approval were obtained from the Ethics Committee of the relevant university before starting the research (number: E-39243114-770-67670/31/04). The purpose of the research was explained to the participants, and their written and verbal consent was obtained.
4. Results
Demographic data of the students in the study are given in Table 1. Of the participants, 58.5% were male, 49.2% lived in the city, 79.1% had a nuclear family, 83.1% had no job, and 49.5% had income equal to their expenses. It was determined that 96% were single, and 62.8% were studying in the first grade. It was determined that 89.7% of the students participating in the study had the COVID-19 vaccine certificate, 40.2% had received expert information about the vaccine, and 22.9% had a positive opinion about the vaccine. Also, 86.7% of vaccinated students had BioNTech/Pfizer, 72.96% had two doses of vaccine, and 40.5% had no side effects. Besides, 4.46% applied to a health institution, and 4.46% used non-medicinal herbal products. Moreover, 10.37% of the students felt a change in their bodies after the vaccination, and 3.70% were diagnosed with other diseases (diabetes, blood pressure, etc.). The side effects seen in the students after the vaccine, the duration of the side effects, and any diagnosis status are given in Table 2.
| Features | Values; No. (%) or Mean ± SD |
|---|---|
| Gender | |
| Woman | 250 (41.50) |
| Man | 352 (58.50) |
| Age | 20.51 ± 2.94 |
| Place of residence | |
| Village-district | 98 (16.3) |
| City | 296 (49.2) |
| Big city | 208 (34.6) |
| Family form | |
| Nuclear family | 476 (79.1) |
| Extended family | 126 (20.9) |
| Working status | |
| Employed | 102 (16.7) |
| Inoperative | 500 (83.1) |
| Income status | |
| Income lower than expenses | 256 (42.5) |
| Income equal to expenses | 300 (49.8) |
| Income more than expenses | 46 (7.6) |
| Marital status | |
| Single | 578 (96) |
| Married | 24 (4.0) |
| Class | |
| First class | 378 (62.8) |
| Second class | 180 (29.9) |
| Third class | 20 (3.3) |
| Fourth class | 24 (4.0) |
Demographic Data
| Variables | Values; No. (%) |
|---|---|
| Side effects after COVID-19 vaccine | |
| No | 244 (40.5) |
| Pain | 132 (21.9) |
| Dizziness | 136 (22.6) |
| Weakness | 20 (3.3) |
| Fire | 6 (1.0) |
| Nausea | 2 (0.3) |
| Hair loss | 2 (0.3) |
| Sleeping state | 4 (0.7) |
| Heart stuck feeling | 6 (1.0) |
| Nausea | 0 (0) |
| Pain + dizziness | 2 (0.3) |
| Pain + fatigue | 14 (2.3) |
| Pain + fever | 4 (0.7) |
| Pain + nausea | 4 (0.7) |
| Dizziness + fatigue | 2 (0.3) |
| Dizziness + nausea | 2 (0.3) |
| Fatigue + fever | 10 (1.7) |
| Heart compression + nausea | 2 (0.3) |
| Pain + dizziness + nausea | 2 (0.3) |
| Pain + fatigue + fever | 6 (1.0) |
| Duration of side effects after vaccination (days) | |
| 1 | 76 (21.23) |
| 2 | 148 (41.34) |
| 3 | 54 (15.08) |
| 4 | 12 (3.35) |
| 5 | 12 (3.35) |
| 6 | 2 (0.56) |
| 7 | 46 (12.85) |
| 8 or above | 8 (2.23) |
| Receiving any post-vaccine disease diagnosis | |
| Yes | 20 (3.70) |
| No | 520 (96.29) |
The Side Effects in the Students After the Vaccine, the Duration of the Side Effects, and Any Diagnosis Status
The students’ opinions about the COVID-19 vaccine are given in Table 3. Among the students participating in the study, 29.25% of those with the COVID-19 vaccine stated that they felt more resistance to COVID-19. It was determined that 17.77% of them changed their opinion about the vaccine after the vaccination. Also, 30.74% of the participants had COVID-19, and 77.10% of those with COVID-19 had the disease before being vaccinated. Besides, 37.77% of the students said they thought negatively about the vaccine after being vaccinated.
| Variables | Values; No. (%) |
|---|---|
| The state of feeling resistant to disease after vaccination | |
| Yes | 158 (29.25) |
| No | 382 (70.74) |
| Post-vaccine opinion change status | |
| Yes | 96 (17.77) |
| No | 444 (82.22) |
| Previous COVID-19 status | |
| Yes | 166 (30.74) |
| No | 374 (69.25) |
| Time to have COVID-19 | |
| Before vaccination | 128 (77.10) |
| Between vaccine doses | 18 (10.84) |
| After vaccination | 20 (12.06) |
| Negative thinking about the vaccine after being vaccinated | |
| Yes | 204 (37.77) |
| No | 336 (62.22) |
| The situation of people who have negative opinions about the vaccine | |
| Yes | 216 (40) |
| No | 324 (60) |
| Current opinion on the vaccine | |
| Positive | 30 (5.0) |
| Negative | 216 (35.9) |
| No idea | 356 (59.1) |
Opinion on the COVID-19 Vaccine
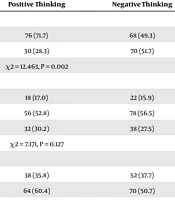
The factors affecting students’ opinions about the COVID-19 vaccine are given in Table 4. It was determined that the gender of the students included in the study significantly affected their opinions about the vaccine. The majority of those with positive opinions about the vaccine were male students (71.7%) (P = 0.002). The status of getting expert information about the vaccine affected the students’ opinions about the vaccine statistically, and the majority of those who had no idea about the vaccine did not receive expert information (P < 0.001). The obsession with having COVID-19 affected the opinions about the vaccine statistically significantly, and the majority of those who had a negative opinion about the vaccine were those who had not had COVID-19 (P = 0.003). The income level of the students had a statistically significant effect on their views on vaccination. The difference was due to the students whose income was higher than their expenses (P = 0.023). The place of residence, class, and the expert from whom information was obtained (doctor/nurse/health worker) did not affect opinions about the vaccine (P = 0.127, P = 0.09, and P = 0.159).
| Categories | Opinions About the Vaccine | ||
|---|---|---|---|
| No Idea | Positive Thinking | Negative Thinking | |
| Gender | |||
| Man | 208 (58. 1) | 76 (71.7) | 68 (49.3) |
| Woman | 150 (41.9) | 30 (28.3) | 70 (51.7) |
| Test, P | χ2 = 12.463, P = 0.002 | ||
| Place of residence | |||
| Rural | 58 (16.2) | 18 (17.0) | 22 (15.9) |
| City | 162 (45.3) | 56 (52.8) | 78 (56.5) |
| Big city | 138 (38.5) | 32 (30.2) | 38 (27.5) |
| Test, P | χ2 = 7.171, P = 0.127 | ||
| Income status | |||
| Income less than expenses | 166 (46.4) | 38 (35.8) | 52 (37.7) |
| Income equal to expenses | 166 (46.4) | 64 (60.4) | 70 (50.7) |
| Income higher than expenses | 26 (7.3) | 4 (3.8) | 16 (11.6) |
| Test, P | χ2 = 11.330, P = 0.023 | ||
| Class | |||
| First class | 242 (67.6) | 62 (58.5) | 74 (53.6) |
| Second class | 96 (26.8) | 34 (32.1) | 50 (36.2) |
| Third class | 6 (1.7) | 4 (3.8) | 10 (7.2) |
| Fourth class | 14 (3.9) | 6 (5.7) | 4 (2.9) |
| Test, P | χ2 = 17.176, P = 0.09 | ||
| Status of receiving expert information | |||
| Area | 118 (33.0) | 50 (47.2) | 74 (53.6) |
| Not received | 240 (67.0) | 56 (52.8) | 64 (46.4) |
| Test, P | χ2 = 20.290, P < 0.001 | ||
| Source of information | |||
| Doctor | 46 (52.3) | 34 (60.7) | 34 (51.5) |
| Nurse | 22 (25.0) | 16 (28.6) | 6 (9.1) |
| Health employee | 20 (22.7) | 6 (10.7) | 26 (39.4) |
| Test, P | χ2 = 3.682, P = 0.159 | ||
| Sickness status | |||
| Passed | 96 (26.8) | 42 (39.6) | 28 (20.3) |
| Did not pass | 262 (73.2) | 64 (60.4) | 110 (79.7) |
| Test, P | χ2 = 11.475, P = 0.003 | ||
Distribution of Some Characteristics of the Students According to Their Opinion About Vaccination (n = 602)
5. Discussion
This study aimed to determine the vaccination status of university students and their opinions about the vaccine regarding the COVID-19 vaccination activities carried out in Turkey.
In our study, 89.7% of the students were vaccinated. According to the Turkish Ministry of Health data, the rate of getting at least two vaccination doses was 85.65%. However, some people are against the COVID-19 vaccine in Turkey. Tucker et al. stated that 29.6% of young people aged 18 - 25 had been vaccinated, while Okamoto et al. reported that 77.3% of young university students had been vaccinated. It is seen that the vaccination rate of the students included in our study is higher than in the literature. It is an opinion that this may be due to the widespread use of vaccination throughout the country and the fact that the order of vaccination has decreased over time to the younger age group (12, 13). The World Health Organization recommends two doses of the COVID-19 vaccine. In our study, 72.96% of the students had two vaccine doses. Okamoto et al. (13) stated that 77.3% of the participants had one dose, and 76.5% had two doses of the COVID-19 vaccine. Although this result is compatible with the literature, it can be said that the recommendations of the World Health Organization and the Turkish Ministry of Health are taken into account by the youth in Turkey.
It was determined that 30.74% of the participants in the study had COVID-19 (based on PCR), and the majority of those who had COVID-19 (77.1%) had the disease before they were vaccinated; also, the rates of disease decreased between vaccination doses and after vaccination. In a study, the vaccine’s effectiveness decreased even after six months, but its protection continued (14). Another study emphasized that vaccination reduces the risk of contracting COVID-19 (15). In our study, COVID-19 vaccines were effective in preventing the disease. In this study, participants who received expert information about the vaccine constituted 40.2% of all participants. It was determined that less than half of the participants received expert information. However, those who did not receive expert information did not have an idea about the vaccine at a higher rate than those who received it. A study stated that the level of information was not associated with a positive attitude toward the COVID-19 vaccine (16). However, there are also study results showing that people who receive information about the vaccine have a more positive opinion about the vaccine (17-21).
However, as with all vaccines, prejudice against vaccination was experienced during the COVID-19 pandemic. For this reason, in our country, information was given with the contents prepared by the experts about the vaccine, especially in first-degree health institutions, public service announcements, and TV-radio broadcasts. The fact that most of the young people participating in the research stated that they did not receive expert information shows that they could not benefit from the mass media and the information provided by health institutions. However, it can be an opinion that most of the young people in our study did not receive expert information about the vaccine, which caused them to hesitate about it. The fact that more than half of the young people in our study stated that they were hesitant about the vaccine may be an indication that they need more information. In a study, 45.3% of the participants were hesitant to get vaccinated (22). Another study emphasized vaccine hesitancy among university students (23). Our study is compatible with the literature. Although vaccination rates have increased significantly in our country, students still hesitate about vaccination (24, 25). In this study, the income level affected dissent to the vaccine. Ozderenol and Seboly (26), Lim and Pranata (27), and Velazquez et al. (28) stated that the effectiveness of the vaccine might vary according to income level, lifestyle, and chronic disease status, which may affect the opinion about the vaccine. Our study findings are compatible with the literature.
5.1. Conclusions
In this study, most young people were vaccinated, although more than half were hesitant about the vaccine. Opinions about the vaccine are affected by some sociodemographic characteristics and the availability of expert information. Therefore, it is essential to inform young people about vaccination. In addition, the necessary information should be given to both health institutions and public health centers about the vaccine, and the public should be informed about vaccines.
Limitation of the study: The study was conducted with participants in the young age group studying at a state university in the central Anatolian region. It cannot be generalized to society. It is recommended that the study be carried out with wide participation and in different regions.