1. Background
Rituximab is a monoclonal antibody against CD20 antigen present on mature B lymphocytes (1-3). It acts through a combination of cell-dependent cytotoxicity, complement-dependent pathways, induction of apoptosis, and ultimately selective reduction of CD20-positive B lymphocyte subgroups (2, 3). Therefore, it is used to treat autoimmune disorders caused by an abnormal increase in the number of B cells and B cells hyperactivity or dysfunction (4). The humoral immune system is suppressed a few months after the start of the drug and may result in bone marrow suppression (5). Rituximab is commonly used in some hematologic and rheumatologic diseases (3). It is also used in some neurological disorders, including multiple sclerosis (MS) and neuromyelitis optica (NMO) (1).
In NMO disease, rituximab has become a standard treatment (6). Rituximab may also be used in the treatment of progressive MS where treatment options are limited. It can also be used in relapsing-remitting multiple sclerosis (RRMS) when it does not respond to common and approved drugs (7).
Studies have shown a significant decrease in the number of new attacks and brain lesions in patients with RRMS, following the administration of rituximab (2, 5, 8). Despite promising results of studies as well as the cost-effectiveness of administration of rituximab, this drug has not yet been approved for the treatment of MS and is used only as an off-label in cases where it has indication (8). Treatment with off-label agents is limited to physicians' personal experiences or small clinical trials, and there are no specific guidelines for this type of treatment (dose and administration interval) (1).
Another issue is the safety and side effects of this drug, which has not been studied systematically, and there is limited experience in this regard (1). Some studies have been conducted on the side effects of this drug in other diseases such as rheumatoid arthritis (RA), but due to differences in patient population and drug administration regimens, its generalization to MS patients should be considered cautiously (1, 9).
In a review article published in 2012 by Kasi et al., severe side effects related to rituximab have been collected from several articles. This study only focused on severe side effects and included cases that could lead to death, hospitalization, severe or permanent disability, or requiring interventional actions. Overall, this study found that the most common side effects in 80-90% of studies were infusion reactions, including anaphylaxis or allergic reactions such as hoarseness, sneezing, dyspnea, and respiratory failure with or without changes in blood pressure or cardiac arrhythmia (3).
Another side effect was associated with cytopenia (mainly in the form of lymphopenia), which occurred in combination with chemotherapy drugs. Increased incidence of infectious side effects was observed up to one year after treatment, but all the patients have been significantly improved without sequelae. Older people were exposed to a higher risk of infectious side effects. The cardiac side effects reported in this article include supraventricular arrhythmias, tachycardia, and rare cases of myocardial infarction, tamponade, and heart failure. Cases with pulmonary side effects such as infection, interstitial lung disease, and rare cases of status asthmaticus and diffuse alveolar hemorrhage have also been reported.
Neoplastic side effects such as leukemia and myelodysplastic syndrome, gastrointestinal disorders such as intestinal obstruction and even intestinal rupture, and ultimately rare cases of neurological manifestations such as seizures, cerebral infarction and serotonin syndrome are also mentioned in this article. It is notable that most of the studies reviewed in this article have focused on patients other than MS and NMO patients (3).
Overall, although rituximab has not been approved for use in MS treatment, its efficacy, especially in RRMS cases, has been shown in studies, and its use as an off-label in MS treatment is increasingly expanding. Although there are some data about the safety of rituximab in MS and NMO patients, there are few experiences regarding its safety in the Iranian population with MS, and known complications are often related to the studies conducted on the patients in other countries or with other autoimmune diseases and malignancies that are different from the population of patients with MS in terms of the underlying disease status and the concomitant medications or previous medications used. Therefore, due to the limited number of studies in this field, especially in Iran, we aimed to conduct this study.
2. Objectives
The aim of this study was to investigate the infusion related, short-term and delayed side effects of rituximab in patients with MS and NMO in the population of the south of Iran.
3. Methods
This was a longitudinal study conducted retrospectively and prospectively at the same time. In this survey, patients with MS and NMO have been studied since the first infusion of the drug and the side effects of rituximab were evaluated.
The study population consisted of patients with MS and NMO who were referred to the health centers affiliated to Shiraz University of Medical Sciences from September 2018 to February 2019 and received Rituximab.
Inclusion criteria were all patients with a definitive diagnosis of MS or NMO based on 2017 McDonald criteria (10) and 2015 NMO diagnostic criteria (11) that were nominated by their treating neurologist to receive rituximab and had no contraindication for taking the drug. Exclusion criteria included uncertainty of diagnosis, presence of concomitant diseases such as rheumatic or hematologic diseases or malignancies that are independently indicative of receiving rituximab, and patient's failure to respond in telephone follow-ups.
A checklist prepared by the researcher was used to collect the data. It consisted of three parts. The first part included the demographic and clinical characteristics of patients, the second part was about the relevant clinical side effects during drug administration and the third part dealt with recoding the delayed clinically apparent side effects of taking rituximab. The endpoint of the study mainly focused on clinical manifestations of the side effects. Thus, the only recorded data in the checklist were the change in the health status of the patients. The first and second parts of the checklist were completed for each patient at the time of entry into the study and receiving rituximab. The group of patients receiving their first infusion of rituximab was prospectively followed up by telephone, one month, four months, and six months after the first infusion, and the third part of each checklist was completed for them.
In other patients who had more than one infusion, information about the first infusion and the side effects produced until then was retrospectively obtained. The side effects produced during the first month after drug infusion were considered as short-term side effects, and the side effects developed after one month were considered as delayed side effects. Each of these manifestations was considered to be a drug-induced side effect if they were not present before starting the medicine and had no secondary cause.
All patients were on biosimilar product (Zytux, AryoGen Pharmed, Tehran, Iran) of MabThera (Rituximab, Roche). The prescribed dose of rituximab in all patients was 1,000 mg, which was administered in 500 mL of normal saline. In the first dose, the infusion was started at a rate of 50 mg/h, and the infusion rate increased every 30 minutes at the same rate to reach a maximum rate of 400 mg/h. In the subsequent doses, the infusion was started at a rate of 100 mg/h and then increased every 30 minutes at the same rate to reach a maximum rate of 400 mg/h. All patients were given 2 mg clemastine, and 100 mg hydrocortisone by infusion and 500 mg acetaminophen orally before rituximab administration, and all patients underwent cardiac monitoring and regular blood pressure check during drug infusion. In the NMO patients, the first two doses of the drug were administered two weeks apart.
After data collection, the data were entered into SPSS software, version 22. Descriptive data were expressed in tables and diagrams (mean and standard deviation), and statistical tests, including independent t-test and chi-square test, were used for data analysis. The significance level was considered 0.05 in this study. An informed consent form was completed for each patient at the time of entry into the study, and patient information was kept confidential. This study was approved by the Iran National Committee for Ethics in Biomedical Research with the registration number IR.SUMS.REC.1397.932.
4. Results
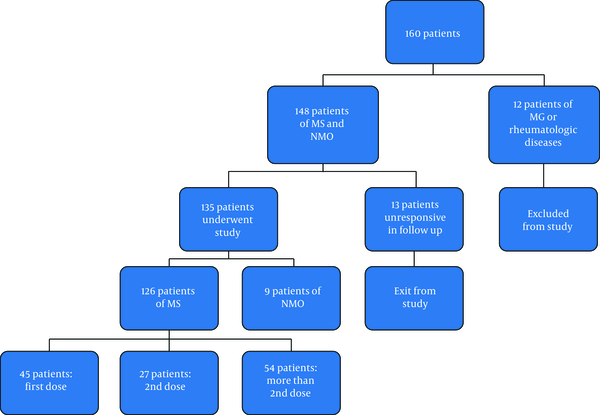
During the specified period, a total of 160 patients were referred to the mentioned centers for receiving rituximab (Figure 1).
Of 135 patients, 95 (70.4%) were female and 40 (29.6%) were male. In terms of age, the mean age of the patients with MS was 36.7 years, the youngest patient was 11 years, and the oldest patient was 66 years old. The highest frequency was in the age group of 31 - 40 years. In patients with NMO, the mean age was 41.44 years; the youngest age was 23, and the highest age was 57 years.
Accordingly, our patients were divided into four groups:
Group 1: patients with MS who were included in the study when receiving the first dose of the drug and were, in fact the same group who were prospectively followed up (45 patients).
Group 2: patients with MS who were included in the study when receiving the second infusion and received their first dose 6 months ago (27 patients).
Group 3: A group of patients with MS who were infused more than two times (54 patients).
Group 4: patients with NMO (9 patients).
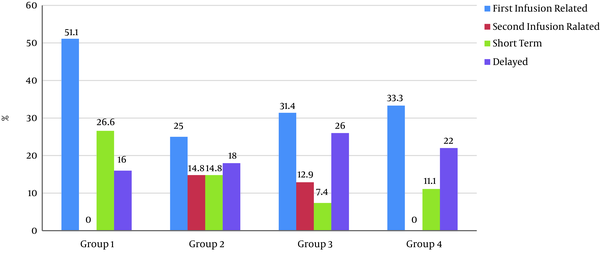
Figure 2 illustrates the distribution of all categories of side effects in the patient groups.
4.1. First Infusion-Related Reactions
Among all patients, 50 (37%) had at least one side effect during the first infusion, which is described in Figure 2, according to different groups. The most common side effect developed during the first infusion was chills (shivering), which occurred in 22 patients (16.3%). Other side effects are shown in Table 1. Regarding the difference in the frequency of the incidence of side effects between the four groups of patients, the relationship between the group and the incidence of side effects was not statistically significant (PV 0.136) (Table 2). Among the side effects developed during the first infusion, receiving the full dose of the drug was not possible only in one case, due to the incidence of severe bradycardia. In other patients, the side effect was resolved by temporary discontinuation of the drug and initiation at a lower rate.
| Infusion Reaction in 1st Injection | Infusion Reaction in 2nd and More Injections | Short-term Side Effects | Delayed Side Effects | |
|---|---|---|---|---|
| Chills | 22 (16.3) | 1 (1.1) | 2 (1.5) | 2 (1.5) |
| Throat irritation, cough | 16 (11.9) | 5 (5.5) | 1 (0.7) | - |
| Dyspnea | 13 (9.6) | 3 (3.3) | 3 (2.2) | 1 (0.7) |
| Cardiovascular | 13 (9.6) b | 1 (1.1) c | 1 (0.7) d | 2 (1.5) e |
| Cutaneous f | 10 (7.5) | 1 (1.1) | 7 (5.5) | 8 (5.9) |
| Fever | 10 (7.5) | 1 (1.1) | 1 (0.7) | 2 (1.5) |
| Dizziness | 5 (3.7) | 2 (2.2) | 1 (0.7) | - |
| Nausea | 4 (3) | 3 (3.3) | 1 (0.7) | 1 (0.7) |
| Headache | 4 (3) | - | 4 (3) | 3 (2.2) |
| Myalgia, malaise | 2 (1.5) | 1 (1.1) | 6 (4.4) | - |
| Anorexia | - | - | 1 (0.7) | 3 (2.2) |
| Abdominal pain | - | - | 1 (0.7) | - |
| Infections g | - | - | - | 7 (5.2) |
| Hair loss | - | - | - | 5 (3.7) |
| Menstrual irregularity | - | - | - | 2 (1.5) |
| Weight loss | - | - | - | 2 (1.5) |
| Weight gain | - | - | - | 2 (1.5) |
Type and Frequency of Each Category of Side Effects a
| Infusion Reaction in 1st Injection | Infusion Reaction in 2nd And More Injections | Early Side Effects | Late Side Effects | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P-Value | Yes | No | P-Value | Yes | No | P-Value | Yes | No | P-Value | |
| Age a | 35.5 (9) | 37.8 (10) | 0.2 b | 39.9 (11) | 38.5 (9) | 0.65 b | 39.2 (7) | 36.5 (10) | 0.27 b | 38.4 (9) | 36.5 (10) | 0.39 b |
| Gender c | 0.047 d | 0.09 d | 0.68 d | 0.125 d | ||||||||
| Female | 41 (80.4) | 54 (64.3) | 10 (90.9) | 52 (65.8) | 14 (66.7) | 81 (71.1) | 23 (82.1) | 72 (67.3) | ||||
| Male | 10 (19.6) | 30 (35.7) | 1 (9.1) | 27 (34.2) | 7 (33.3) | 33 (28.9) | 5 (17.9) | 35 (32.7) | ||||
| Groups c | 0.13 d | 0.71 e | 0.07 d | 0.63 d | ||||||||
| Group 1 | 23 (48) | 22 (27.9) | - | - | 12 (60) | 33 (31.1) | 7 (26.9) | 38 (38) | ||||
| Group 2 | 7 (14.8) | 20 (25.3) | 4 (36.3) | 23 (32.9) | 4 (20) | 23 (21.7) | 5 (19.2) | 22 (22) | ||||
| Group 3 | 17 (36.1) | 37 (46.8) | 7 (63.6) | 47 (67.1) | 4 (20) | 50 (47.2) | 14 (53.8) | 40 (40) | ||||
Evaluation of the Relationship Between Each Category of Side Effects with Age, Gender, and Patient Groups
4.2. Side Effects During the Second Infusion and the Subsequent Ones (Next Infusions)
Side effects during the second infusion and the subsequent infusions were reported in 11 patients (13.5%) in groups 2 and 3. These side effects were not observed in any of the patients with NMO (Figure 2). The most common side effect developed during the second infusion was throat irritation and burning observed in 5 (5.5%) of all patients. Other side effects are shown in Table 1.
4.3. Comparison Between the Incidence of Infusion Reactions During the First Fusion and the Subsequent Ones
We compared the first and next infusions to investigate at what stage the side effects are more frequent. In total, among the subjects in groups 2, 3, and 4 who received two or more infusions, 27 patients experienced the side effects during the first infusion and 11 patients experienced the side effects during the next infusions. In comparison of the side effects developed during the first infusion and the next infusions PV 0.004 was obtained, which was statistically significant.
4.4. Short-term Side Effects
Short-term side effects occurred in 21 (15.5%) patients (Figure 2). The most common short-term side effect was skin manifestations (itching, redness, rash, dryness), which were seen in 7 patients (5.2% of all patients). Other side effects are listed in Table 1. These side effects led to hospitalization in two patients, one with fever and chills and the other with abdominal pain. In both cases, despite investigations, no secondary cause was found for the patient's symptoms, and the symptoms resolved spontaneously.
4.5. Delayed Side Effects
Delayed side effect was developed in 28 (20.7%) patients, as shown in Figure 2. The most common delayed side effect developed was skin manifestations (dryness and scaling, itching, and rash) that occurred in 8 patients (5.9%). Other side effects are listed in Table 1. The relationship between the incidence of delayed side effects based on the number of infusions was investigated in MS patients, which was not statistically significant.
The relationship between each of the side effects during the first infusion, during the next infusions, the short-term side effects, and the delayed side effects with age and gender was investigated (Table 2). The relationship between the side effects during the first infusion and gender was significant (PV: 0.04). No relationship was found in other categories. Of all the patients studied, only one was unable to continue taking rituximab and was a candidate for drug replacement due to the incidence of severe and sustained bradycardia at two times of infusion.
5. Discussion
5.1. Demographic Characteristics
In terms of gender distribution, the higher proportion of women in this study was similar to the most previous studies due to the higher prevalence of the disease in women. In terms of mean age, it was almost similar to other studies as well.
5.2. Infusion-Related Reactions
Of the four categories of side effects investigated (first infusion-related reactions, next/subsequent infusions related reactions, short-term side effects, and delayed side effects), the most common side effect was the “first infusion-related reactions” that occurred in 37% of the patients. In the study of Hauser et al. (2), infusion-related reactions were reported in 78.3% of patients and Hawker et al.’s study (12), it was reported in 67.1% of patients, both of which were higher than those in our study. However, these side effects were seen in only 7.8% of the 822 patients in the study of Salzer et al. (13). Also, in Scotti et al. (8) study, these side effects were seen in only 10 out of 339 infusions. The incidence of side effects was lower in these two studies than in our study.
There are two notable points in this regard. The first point is that our patients had all received premedication with corticosteroids, antihistamines, and acetaminophen prior to the infusion of rituximab. In the study of Hauser et al. (2), it was noted that none of the patients received premedication. However, in the other three studies, there was no evidence that whether premedication was used or not. The second point is that both studies in which side effects during infusion were higher were carried out prospectively, but the two other studies were conducted retrospectively and based on the previously recorded data.
Among the four groups of patients, the side effects during the first infusion, most commonly accured in group 1. Group 1 consisted of the same patients who were prospectively followed, while in patients in groups 2 and 3, information about the first infusion was received retrospectively, which increases the possibility of error in the recall. Despite this difference between the frequency of side effects in different groups of patients with MS, no significant relationship was found between the group and the incidence of side effects by doing the statistical test. Side effects during the first infusion occurred in women more than men, indicating a statistically significant relationship.
The most common side effect during infusion was chills (shivering) followed by throat irritation and burning which had a high frequency in the studies of Hauser et al. (2) and Hawker et al. (12) as well. The incidence of cardiovascular side effects (tachycardia, bradycardia, hypertension, and hypotension) was higher in our study than in these two studies, but in another study (3), the incidence of severe cardiovascular side effects (grades 3 and 4) was reported to be 8%. Of the side effects during infusion, receiving the full dose of the drug was not possible only in one case with severe bradycardia. In other cases, side effects were resolved by temporarily interrupting the infusion or slowing down the infusion rate. The incidence of side effects during the next infusions (second infusion and the subsequent ones) was significantly less than the first infusion, indicating a statistically significant difference. This finding is consistent with those of other studies. Cardiovascular side effects were also lower in the subsequent/next infusions than in the first one (13 vs. 1). In the subsequent infusions, bradycardia had only occurred in one patient who was the same patient suffering from bradycardia in the first infusion.
5.3. Short-term and Delayed Side Effects
Short-term side effects (side effects occurred within the first month after infusion), like the first infusion-related side effects, were more prevalent in patients in group 1, which may be attributed to the greater accuracy of the study in this group due to its prospective status. Delayed side effects, unlike the previous category, were more common in patients in group 3 (patients with more than 2 infusions), which may be expected due to the greater frequency of infusion in this group. However, in investigations conducted using statistical tests, no significant relationship was found between MS groups and the incidence of this category of side effects. The most common delayed side effect was cutaneous side effects followed by infectious ones. Short-term and delayed side effects have been considered non-infusion-related side effects in other studies (regardless of the interval from infusion) and their types and frequency have varied in different studies.
5.4. Comparison of Other Results Conducted on Iranian Population
There are few studies regarding rituximab side effects on Iranian patients. In one study, the effects of rituximab in pemphigus was reported (14). Furthermore, Seyed Ahadi et al. and Shaygannejad et al. evaluated the efficacy and safety of rituximab in patients with NMO. In the first study, which was done on different doses of rituximab, the most prevalent side effects were minor infections. But in the second one, infusion reactions were more common that was similar to our study (15, 16). In 2019, Moghaddasi et al. reported their data about rituximab, which mainly focused on the term of efficacy rather than safety. In their survey, the most common side effects were infusion reactions as in our study. However, the prevalence rate was 70% that was higher than ours. It is notable that in that study, there was no evidence that whether premedication was used or not (17).
5.5. Comparison of the Results with Patients with Other Diseases
Numerous previous studies have investigated the side effects of rituximab in other diseases such as malignancies (mainly hematologic malignancies, including non-Hodgkin's lymphoma) and rheumatoid arthritis (RA). According to research articles related to oncology (18), the incidence of side effects during the first infusion was observed in approximately one-third of these patients and decreased in the subsequent infusions. For RA, side effects during the first infusion also occurred in 25% of the patients in one study (18) and 30 - 35% in another study (19), despite receiving corticosteroids as premedication. This incidence rate of side effects during the first infusion was similar to our study, and it seems that there was no significant difference among the three groups. In all three groups, most of the side effects developed were mild to moderate.
According to a study conducted on patients with RA (18), the most common manifestations during infusion were headache, itching, throat irritation and burning, hypertension and fever, which were commonly seen in our study. Studies conducted on patients with malignancy have reported a variety of side effects that have not been seen in patients with RA and MS, including in our study, such as tumor lysis syndrome (3), which may occur in patients with high cell density tumors and cause acute renal failure. Cytokine release syndrome (3, 20) has also been reported in patients with tumors with high cell density after rituximab infusion. In one study (20) conducted on 166 patients with non-Hodgkin's lymphoma, infectious side effects were seen in 30% of the patients, which was higher than that of our study. Most of them, however, were mild.
According to the results of another study (19), blood cell reduction was seen in 48% of patients with non-Hodgkin's lymphoma treated with rituximab, and also in a study (19) delayed neutropenia was reported in 8% of patients up to one year after treatment with rituximab. In our study, this could not be assessed due to the inability to perform laboratory studies. Moreover, In a study carried out on patients with RA (19), less than 1% of patients had severe side effects, leading to discontinuation of medication and inability to continue medication. This occurred in 1 out of 135 patients in our study.
5.6. Disadvantages and Problems
In this study, it was not possible to compare patients with MS and NMO due to the low number of patients with NMO. Other problems with this study included the retrospective part of the disease where there was a possibility of error in the recall. Another problem was the inability to perform laboratory studies to evaluate hematologic side effects such as lymphopenia and anemia that have been reported in some studies. Regarding the duration of follow-up of patients, short-term and mid-term side effects were investigated in our study, and there was no possibility of investigating the long-term side effects (such as malignancies) observed in some previous studies.
5.7. Conclusion
Overall, there were no unexpected side effects in patients under the study and the side effects developed were comparable to previous studies as well as those conducted on patients other than patients with MS and NMO. Side effects developed were often mild, with only two cases, leading to hospitalization, both of which were self-limiting as well, and there were no serious life-threatening side effects, except for one case with bradycardia.