Dear Editor,
Hyper-eosinophilic syndrome (HES) is defined by the presence of hypereosinophilia (blood eosinophilic count of > 1500 cells/µL and/or tissue hypereosinophilia) persisting for longer than one month; no identifiable etiology for eosinophilia and organ damage is directly attributable to the overproduction of eosinophils (1). In spite of the significant progress in the understanding of HES pathogenesis, our knowledge is insufficient for formulating a new comprehensive definition of HES based on its etiology (2). Erythroderma is an uncommon disorder that has a broad differential diagnosis and requires a high index of suspicion for treatment. Its varied underlying etiologies are in need of significantly different approaches to its treatment (3). To the best of our knowledge, only four cases of HES with erythroderma or cutaneous damage in adults have been previously reported in the literature (4-6). Indeed, no HES cases with hydrocephalus had been previously reported. We presented the first Iranian case of HES associated with hydrocephalus and erythroderma.
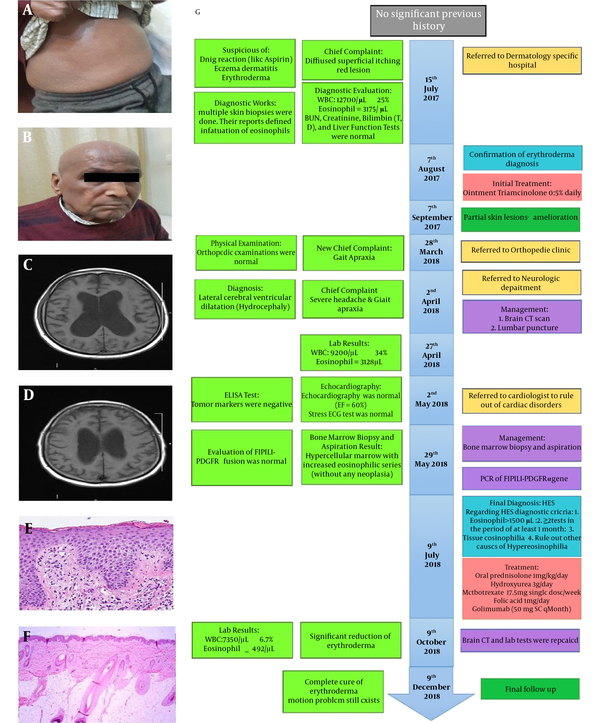
The patient was a 75-year-old Iranian man who presented flat, itchy, and painless red lesions on his trunk since July 2017. After visiting our hospital, complete blood count (CBC), differential blood count, liver function test (LFT), total and direct bilirubin, blood urea nitrogen (BUN), and creatinine (Cr) tests were performed. The results showed eosinophilia in CBC with 3175 counts and total serum immunoglobulin E (IgE) levels (41.9 IU/mL; normal range 173), but the rest of the tests were normal. Then, a skin biopsy was performed from his trunk lesions, which showed eosinophils infiltration. After assessing the results, the dermatologist diagnosed him with erythroderma (Figure 1A and B) and prescribed a daily topical triamcinolone 0.5% for his lesions (7), which somewhat improved his skin lesions.
Clinical data of a 75-year-old Iranian man. A, B, Redness of the patient's entire body caused by erythroderma. C, D, Severe hydrocephalus before the treatment depicted via the brain CT. E, F, Skin biopsies taken from the erythroderma and an increased number of eosinophils reveals surrounding connective tissue before treatment and decreased number of eosinophils after treatment (haematoxylin and eosin, original magnification × 100). G, The algorithm of our diagnostic process.
Six months later, the patient suddenly developed motion problem, and on examination, gate apraxia was found, and brain computed tomography (CT) showed dilation of the lateral ventricles (hydrocephalus). Following three times lumbar puncture procedure, the results of the LP fluid analysis were found to be normal; however, his motion problem did not recover (normal pressure hydrocephalus). A CBC test was performed, and 3128 counts of eosinophilia were discovered. Finally, one month after the rejection of other causes, the patient was diagnosed with HES based on the available evidence and eosinophilic counts of more than 1500 cells/µL. The patient took oral prednisolone (1 mg/kg/day) (8). However, this treatment did not result in his dramatic response; hence, we added hydroxyurea (3 g/day) (9, 10) accompanied by MTX (17.5 mg single weekly dosage), folic Acid (1 mg/day), and golimumab (50 mg SC qMonth) (11). Two months later, his motion problem symptoms improved, and it led to a gradual reduction of redness in his skin. After taking brain CT, dilation of the cerebral ventricles had reduced. The brain CT scan showed lateral ventricular dilation (hydrocephalus) before treatment (Figure 1C) and decreased hydrocephalus after treatment (Figure 1D). Skin biopsies taken from the erythroderma before treatment showed hypereosinophilia surrounding connective tissue (Figure 1E) and decreased number of eosinophils after treatment (Figure 1F).
Classification of HES, including lymphocytic HES, myeloproliferative HES, and idiopathic HES, is defined by eosinophilia in the blood and tissues or both (Table 1) (8). In the present case, all the causes of hypereosinophilia were ruled out. Thus, we tagged our patient in the subclass of idiopathic HES (9).
| Subclasses of HES | Manifestations |
|---|---|
| Myeloproliferative | |
| PDGFRα a-related HES | F/P b mutation defined by RT-PCR and FISH c |
| Unknown etiology | No F/P mutation and clonal eosinophilia defined by HUMARA d PCR at Chromosome X |
| Chronic eosinophilic leukemia | Presence of blasts on peripheral smear and/or cytogenetic anomalies |
| Lymphocytic | |
| PCR of T-cell receptor | Release of Th2 cytokines (like IL-5) |
| Flow cytometry of clonal lymphocytes | Release of Th2 cytokines (like IL-5) |
| Idiopathic | |
| Benign | Without any organ involvements/asymptomatic |
| Episodic | Eosinophilalia with cyclical angioedema |
| Complex | With organ involvement, symptomatic, and not classified as myeloproliferative or lymphocytic HES |
| Signs & symptoms (Clinical manifestations) | |
| General manifestations | A fever of > 38°C, myalgia, arthralgia, asthenia, etc. |
| Skin | Erythroderma. palpable purpura, livedo racemosa, urticaria, mucosal erosions, angioedema, etc. |
| Lung | Allergic asthma, rhinosinusitis, cough, dyspnea, RURI e, pleural effusion, etc. |
| Gastrointestinal | Abdominal pain, vomiting, diarrhea, ischemic colitis, ascites, etc. |
| Neurologic impairment | Vertigo, paresthesia, aphasia, distal polyneuropathy, etc. |
| Heart | Myocarditis, MI f, valvular abnormalities, pericardial effusion, etc. |
| Hematologic disorder | DVT g, anemia, superficial thrombophlebitis, elevation of serum IgE/tryptase/CRP, etc. |
Different Subclasses of Hyper-eosinophilic Syndrome, Their Characterizations, and Manifestations, as Well as Signs and Symptoms
In a previous research (10), diagnostic criteria for HES were presented based on the studies of Chusid et al. as cited in Chen et al. and Simon et al. (2). These criteria include: (1) blood eosinophilic count of ≥ 1500 cells/µL for a period of > six consecutive months, (2) end-organ involvement and tissue eosinophilia, and (3) absence of any other known causes of hypereosinophilia. The clinical manifestations of HES associated with different organ damages are shown in Table 1.
In our case, the first presentation was the cutaneous symptom. At first, he had visited the hospital with a superficial diffused itching red lesions on his trunk. According to the studies of Klion et al. (9) and Chen et al. (10), the differential diagnoses of hypereosinophilia include: (1) parasitic and fungal infectious diseases; (2) oncologic disease; (3) allergic disease; (4) endocrine disorders; (5) particular organ disorders, such as lungs, skin, vascular abnormality (Churg-Strauss syndrome), gastrointestinal symptom (eosinophil-associated gastrointestinal disease); and (6) immunologic diseases, etc.
Cutaneous manifestations of HES required differential diagnosis with urticaria, pruritus sine materia, mycosis fungoides, cutaneous adverse drug reactions, contact dermatitis, and atopic dermatitis. Dermatologists should consider pruritic, erythematous papules, urticaria, angioedema, dermographism, oral and genital ulcers, centrifugal annular erythema, acral bullae, and erythroderma. Histopathologic examination of the skin lesion is usually nonspecific, with viable eosinophilic infiltration (12). For the initial diagnosis of our patient, we ruled out the above-mentioned disorders. Our performance algorithm ruled out clonal HES and confirmed idiopathic HES in our case (Figure 1G) (10). The diagnosis of HES is often delayed due to its pleomorphic dermatological manifestations and insidious evolution. It is thus crucial that in all cases of erythroderma, HES should be considered as a differential diagnosis (12).
Eosinophils have direct cytotoxicity through the local release of toxic substances, including cationic proteins, enzymes, reactive oxygen species, pro-inflammatory cytokines, and arachidonic acid derived factors. The degree of end-organ damage is heterogeneous, and there is often no correlation between the level or duration of eosinophilia and the severity of organ damage (13).
The causes of nervous involvement in HES are poorly understood. However, one of the possible reasons for hydrocephalus in our case could be the increased CSF release because of hypereosinophilia. Basically, eosinophils at the site of inflammation can produce a large number of cytokines and release lipid mediators and granule proteins like neurotoxin. These may affect the ependymal cells in the choroid plexuses of the brain ventricles and produce an excess amount of CSF (10). The titers of serum rheumatoid factors of ANA, ANCA, and dsDNA were negative. Antifungal parasitic immunoglobulin and IgE were measured, which were found to be negative as well. Regarding our management, bone marrow aspiration and biopsy were done for the analyses of cytology and gene mutation. Hypercellular bone marrow was associated with increased eosinophilic series (without any neoplasia). Using RT-PCR and FISH techniques (10), no mutations of FIP1L1-PDGFRα and PDGFRβ were observed, that is, there was no chromosomal deletion of the CHIC2 domain or gene deletion (fusion) of FIP1L1 and PDGFRα on chromosome 4q12.
According to the patient’s CBC/Diff laboratory tests and after ruling out other causes of hypereosinophilia, he was diagnosed with HES, leading to some complications like erythroderma and hydrocephalus. A few months after the treatments, his tests showed a reduction in eosinophil count and alleviated severities of erythroderma complications and the patient’s hydrocephalus.