1. Background
The World Health Organization (WHO) defines Alzheimer's disease (AD) as an unknown etiological neurodegenerative disorder marked by worsening memory and cognitive decline (1). The most widely recognized type of dementia is AD, and its histopathological characteristics include extracellular amyloid-β (Aβ) plaque deposition, which is caused by amyloid precursor protein (APP), presenilin 1, presenilin 2 (2), neurofibrillary tangles, consisting of Tau protein hyperphosphorylated, and neuronal loss (3). Several neurological centers, including the amygdala, thalamus and hippocampus, parietal projections, and entorhinal cortex, influence memory and realizing, of which the hippocampus plays a significant role (4). The hippocampus is a segment of the temporal memory lobe implicated in the pathology of AD (5), whilst its synaptic communication is related to the formation of long-term potentiation (LTP). As a synaptic plasticity model, LTP incorporates neurobiological elements that sustain the hippocampal memory process (6). An electrophysiological phenomenon is made at the synapse site after high-frequency electrical stimulation (tetanus) of the presynaptic neurons. Indeed, incitement of tetanus causes an abrupt and persistent increment in the postsynaptic reaction (hours to days) (5).
Coffee is one of the most consumed beverages globally, and a high proportion of adults consume it as a beverage form (7), while caffeine (1,3,7- trimethylxanthine), a psychostimulant within the coffee, is frequently used in Western countries (8). The therapeutic properties and health benefits of coffee consumption have been studied extensively for diabetes and neurodegenerative diseases (9). Given that disease prevention is often much safer, quicker, less costly, and cheaper than treating the disease when it happens, prophylaxis is seen as the optimal solution.
2. Objectives
Considering the mentioned issues, so far, and to our knowledge, there are no studies released on the impact of brewed coffee (BC) on LTP in a rat model of AD caused by streptozotocin (STZ). As a result, the goal of this research was to explore the effect of sub-chronic administration of CB on LTP in rat models of AD.
3. Methods
3.1. Animals
Thirty-two mature male Wistar rats (220 - 250 g) were selected from the pet care and breeding department of Zahedan University of Medical Sciences (ZAUMS), Zahedan, Iran, and were kept in a room with a controlled temperature (20 ± 2C) and under a light/dark cycle of 12 - 12 hours (lights on at 6:00 a.m. and dark at 6:00 p.m.). In addition, we allowed animals to have access to water and normal laboratory food ad libitum. All experimental procedures were authorized by the Zahedan ethics committee of ZAUMS (IR. ZAUMS. REC.1397.341). All rats were kept in conventional cages (each cage contained four rats) and then were divided into four groups randomly: (1) Sham: Animals were given normal saline (NS) for 21 days, (2) BC: Rats were given coffee for 21 days before the tests, and (3) STZ: Animals were given STZ and subsequently given NS, in a similar time. In the AD group, most neurons will die after injecting toxic substances like STZ into the cerebral ventricles. This causes AD-like behavior to occur in sensorimotor tests (10). 4) BC-STZ: Rats were given BC for 21 days until receiving STZ to induce AD. Rats' brains were removed and immediately placed in a cool saline bath. The right hemisphere of the rat's brain was used for electrophysiological recording, and their left hemisphere was used for histological studies.
3.2. Preparation of BC
We prepared a filtered BC according to the method proposed by Vitaglione et al. (11): At 90ºC, 10 g of coffee powder was placed in the filter paper, and 100 mL of water was added to the coffee. At the gavage time, the BC was always prepared and given to the rats once a day for 221 days, at a dosage of 5.7 mL/kg per day, which is equivalent to eight 50 mL cups of BC (10 percent) a day, being drunk by an adult person (12).
3.3. LTP Recording
We anesthetized rats using ketamine / xylazine (90/ 10 mg/kg, i.p.) and fixed their heads to implant the electrodes in a stereotaxic device. The bipolar metal wire filming (tungsten wire, CFW Co., USA) and stimulating (stainless steel wire, CFW Co., USA) microelectrodes were then implanted into the granular cells of the left hippocampal dentate gyrus (DG) (AP = ± 3.8 mm, ML = ± 3.2 mm, DV = 2.7 mm) and in the perforant pathway (PP) (AP = -7.5 mm, ML = -4 mm, DV = 3.9 mm), respectively. LTP recording was conducted 0.25, 0.5, 1, and 3h after HFS. In order to assess any improvements in the synaptic reaction of the DG, the percent of population spike (PS) amplitude (AMP) and field excitatory postsynaptic potential (fEPSP) slope were measured (13, 14).
3.4. Hippocampus Staining Method
Hippocampal tissues were treated with 10% neutral buffered formalin, whereupon dehydrated in 50 - 100% C2H5OH solutions before being immersed in paraffin. Tissues were dissected as thin as 5 μm, then stained with Hematoxylin and Eosin (H&E) before being examined under a microscope (15).
3.5. Data Analysis
Data are presented as mean ± standard error of the measurement (SEM). To examine the data, a repeated measure analysis of variance (ANOVA) was used, continued by a Tukey's post-hoc test to check the % AMP and % slope (LTP indices) of fEPSPs at different intervals. Statistical significance was then acknowledged at P < 0.05. All statistical analyses were conducted using Prism Graphpad 7.0.
4. Results
4.1. AMP
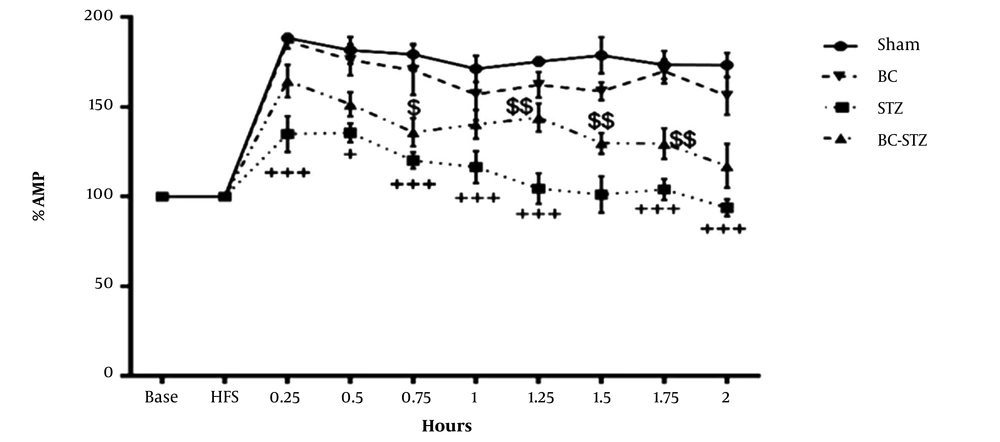
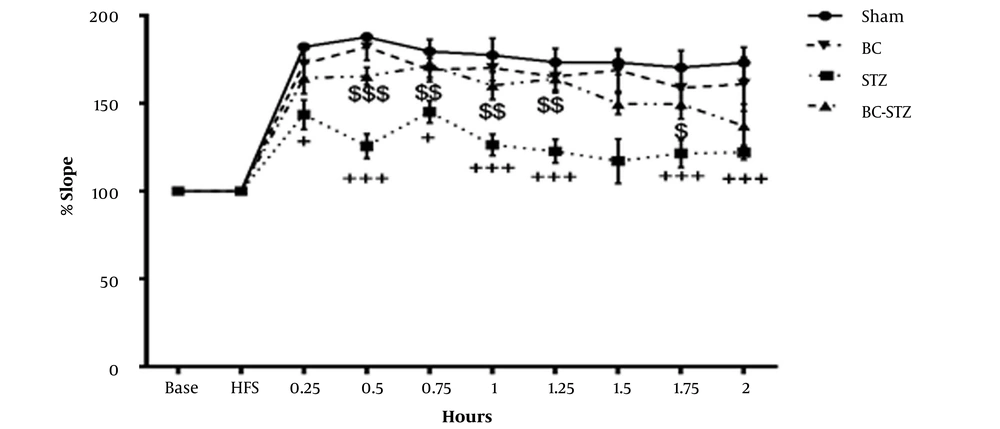
Field potential recordings were obtained after stimulation of the PP in granular cells in the hippocampal DG. In the DG, LTP was caused by the HFS of the PP, and the results of BC pretreatment (before STZ injections) on the percentage of PS AMP and the percentage of EPSP slope and PS AMP of the BC-treated STZ rats are shown in Figures 1 and 2, respectively.
From the dentate gyrus of the hippocampus before and after high-frequency stimulation, the percentage of amplitude (AMP) recorded in the groups according to the mean ± SEM during 0.25, 0.5, 1 and 2 hours after HFS to brain perforant path (PP) was obtained (repeated measures two- way ANOVA followed by HSD post hoc test). + P < 0.05, ++ P < 0.01 and +++ P < 0.001 vs. the sham group and $ P < 0.05, $$ P < 0.01, $$$ P < 0.001 vs. the STZ group.
From the dentate gyrus of the hippocampus before and after high-frequency stimulation, the percentage of slope recorded in the groups according to the mean ± SEM during 0.25, 0.5, 1 and 2 hours after HFS to brain perforant path (PP) was obtained (repeated measures two- way ANOVA followed by HSD post hoc test). + P < 0.05, ++ P < 0.01, and +++ P < 0.001 vs. the sham group and $ P < 0.05, $$ P < 0.01, and $$$ P < 0.001 vs. the STZ group.
The PS AMP was 188.325 ± 10.74% in the sham group and it reduced 134.90 ± 8.58% in the STZ group. The PS AMP in the BC + STZ group (164.23 ± 11.33%;) markedly increased compared to the STZ group 0.25 h after HFS (P = 0.0330). Moreover, PS AMP significantly increased in the BC + STZ group 0.5, 0.75, 1, 1.25, 1.5, 1.75, and 2 h after HFS (P = 0.4481, P = 0.4609, P = 0.1239, P = 0.0017, P = 0.0413, P = 0.0851, and P = 0.1323, respectively).
As can be seen in Figure 1, the LTP also occurred in the STZ group, but its severity was very low compared to other groups. The AMP (%mv) meaningfully increased (P < 0.05 and P < 0.01, respectively) 0.75, 1, 1.25, 1.5, 1.75, and 2 h after HFS in these groups. In addition, there was a meaningful difference between the STZ group compared to the sham group in all LTP recording times after HFS.
4.2. Slope
The EPSP slope was 182.11 ± 4.6% in the sham group. The EPSP slope decreased to 143.62 ± 9.4% (P < 0.05) in the STZ group. However, the oral administration of BC (BC+STZ group) significantly (P < 0.001) increased the hippocampal DG EPSP % slopes 0.5 h after HFS (165.47 ± 12.4%). The BC pretreatment (three weeks) preserved the decrement of the hippocampal EPSP slope caused by the STZ injection 0.75, 1, and 1.25 h after HFS (P = 0.0394, P = 0.0038, and P = 0.0002, respectively) in comparison with the STZ group. There was also a marked difference between the STZ group compared to the sham group in all LTP recording times, except 1.5h after HFS.
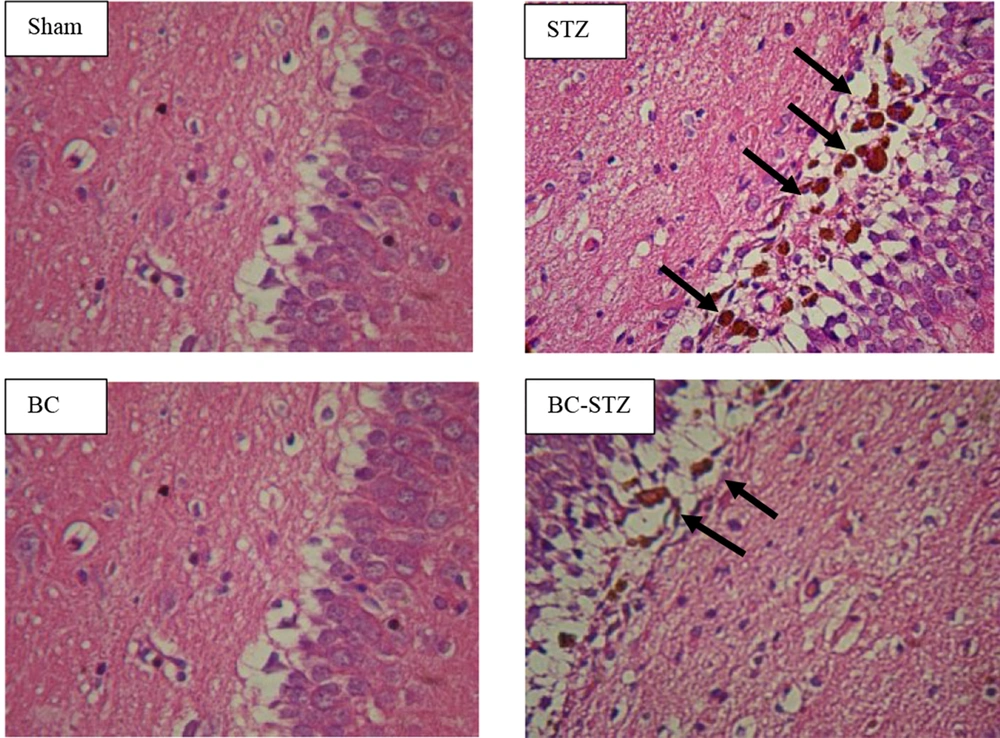
4.3. Histological Evaluation
In the current paper, diffuse plaques were identified in the DG area of the hippocampus ten days following the development of AD, utilizing H&E staining. BC treatment significantly healed the histopathological injury in AD rats (reduced the AD amyloid plaques in DG area of the hippocampus) (Figure 3).
5. Discussion
The goal of the current research was to assess the possible impact of sub-chronic BC administration from roasted coffee beans on LTP after AD induction. Accordingly, we found that coffee administration ameliorated synaptic dysfunction. Hippocampal LTP a long-lasting increase in synaptic efficacy. Numerous studies have indicated that hippocampal LTP is the cellular learning and memory basis (16-18), and is abnormally controlled in various diseases, including AD, Parkinson's disease, and stroke (19-21); thus, control of hippocampal LTP may be a putative method to treat these pathological conditions (22, 23). The BC is one of the world's most popular beverages, consumed regularly by millions of people, and is known as a psychoactive stimulant due to its caffeine content, resulting in increased alertness and excitement and improved cognitive performance (24). Recently, animal studies have suggested that chronic consumption of caffeine could inhibit Aβ production in rodent brains (25), improve the cognitive performance of AD (26), or prohibit a cognitive reduction in male rats (27). Moreover, caffeine has been demonstrated as an antioxidant to reduce oxidative stress (28) and helps to combat blood-brain barrier (BBB) disorders (29).
The AD starts with a synaptic dysfunction, and A2A receptors (A2AR) are mainly found in synapses that control synaptic plasticity. Indeed, A2AR over-activation induces memory deficits, and A2AR blocking hampers memory loss in AD models (30). Espinosa et al. investigated the effect of caffeine on STZ-induced AD and related hippocampal neurodegeneration and also on the expression and density of adenosine receptors. In this regard, adult male rats received a bilateral saline or STZ infusion (3 mg/kg, ICV), which induced memory impairments four weeks later, and led to impaired object recognition memory. This was followed by a decrease in neuronal nucleus antigen (NeuN) immunoreactivity in the hippocampal region of CA1 and improved adenosine A2AR expression and density in the hippocampus, but not A1 receptor (A1R). Accordingly, the investigators observed that the intake of caffeine (1g/L in drinking water two weeks before the STZ challenge) inhibited memory deterioration and neurodegeneration caused by STZ and also A2AR up-regulation (31). Larsson and Orsini showed that there is no association between 1 cup/ day of coffee consumption and the increased risk of overall dementia or AD specifically (32); moderate coffee drinking (3 - 5 cups/day) has a protective role in preventing the risk of dementia (14).
In the present study, the daily dosage was 5.7 mL/kg, which is equivalent to eight 50 mL cups of BC (10%) a day, being drunk by an adult person. Furthermore, a significant improvement in memory was observed, which is in line with the results of Arendash and Cao, who found a significant improvement in memory in 18-to-19-month-old rats after caffeine administration 1.5 mg per day (human equivalence of 500 mg/d) compared to the controls (four weeks: 217%, fifth weeks: 198%) (33). However, a recent meta-analysis revealed that caffeine consumption from coffee or tea has no statistically significant protective effect, while the pattern has decreased the risk of cognitive diseases, such as AD and dementia, by 18% (34). Concerning the present study, there are some limitations that should be considered. In particular, LTP has been reported alone, and biochemical and histological data were not collected due to the lack of facilities. Therefore, more comprehensive studies are required to determine the optimal dose of coffee for cognitive function and thereby yield more conclusive findings.
5.1. Conclusions
In conclusion, the present study indicated that BC consumption improved the synaptic plasticity and thus memory in rat models with AD induced by STZ. However, further studies are required to explain the neuroprotective mechanism attributed to BC.