1. Background
Coronavirus disease 2019 (COVID-19) is sweeping across the globe, resulting in more than 3,059,642 confirmed cases and 211,028 deaths worldwide as of 30 April 2020 (1). The mortality rate of COVID-19 patients varies considerably from 1.4% to 15% (1-5), which may be attributed to pre-existing characteristics such as age, comorbidities, and disease severity. Patients with diabetes have been reported to have higher rates of severe disease and mortality than non-diabetics (2, 3).
As known, COVID-19 and diabetes mellitus (DM) are related in two ways. First, diabetes has been reported to be linked with adverse COVID-19 prognosis. Second, newly diagnosed hyperglycemia and severe complications of pre-existing DM, including diabetic ketoacidosis (DKA) and hyperosmolarity, have been reported in patients with COVID-19 (4, 5).
The COVID-19 virus may enter the pancreatic beta cells by expressing angiotensin-converting enzyme 2 (ACE2) receptors, impairing insulin production and, consequently, either worsening DM or developing new-onset DM (5). High levels of interleukin-6 and tumor necrosis factor-alpha in patients with severe COVID-19 could induce insulin resistance and hyperglycemia (6).
Few, if any, studies are available regarding the frequency of diabetes mellitus, newly diagnosed hyperglycemia, and their impacts on hospitalized patients with COVID-19 (5-8).
2. Objectives
This study aimed to determine the frequency of pre-existing DM and newly diagnosed hyperglycemia among COVID-19 patients and assess the impact of these conditions on the clinical features and severity of COVID-19.
3. Methods
This retrospective study examined the medical records of hospitalized COVID-19 patients admitted to Razi Hospital, Ahvaz, southwestern Iran, from February 2019 to August 2020. We included all medical records of patients with COVID-19 infection (N = 860). Medical records with incomplete information were excluded (n = 50). The COVID-19 infection was confirmed based on reverse transcription-polymerase chain reaction (RT-PCR) of the throat and nasal swab specimens and/or typical chest computed tomography (CT) scan findings, including distributed multifocal ground-glass opacities (GGO) and patchy consolidation in suspected patients (9). The required data were obtained from the history form, clinical chart, nurse notes, physician reports, and laboratory data.
Newly diagnosed hyperglycemia was confirmed in patients without previous diabetes with two or more blood glucose tests > 180 mg/dL and HbA1C less than 6.5% on the first day of hospitalization (2). Also, HbA1C was assessed based on the Immune-Turbidimetry method using the Mancompany kit. Diagnostic criteria for DKA and HHS were based on the American Diabetes Association (ADA) Standards of Medical Care in Diabetes 2019. The earliest day of the serum creatinine change that met the kidney disease improving global outcome (KDIGO) criteria for AKI was selected as day 1 of AKI, with an increase in the serum creatinine of 1.5 times (10). The National Early Warning Score 2 (NEWS2) scoring system was used to determine the severity of the disease (11).
This study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (Ref. ID: IR.AJUMS.REC.1399.493).
3.1. Statistical Analysis
Descriptive statistics such as mean, percentage, and confidence interval were used to describe the data. Analytical statistics, including the chi-square test, were used to compare ratios, and the independent sample t-test was used to compare the mean values between the two groups. The logistic regression model determined the risk factors affecting death and the need for ICU admission in all patients. The collected data were analyzed using SPSS (version 25) software. P values less than 0.05 were considered statistically significant.
4. Results
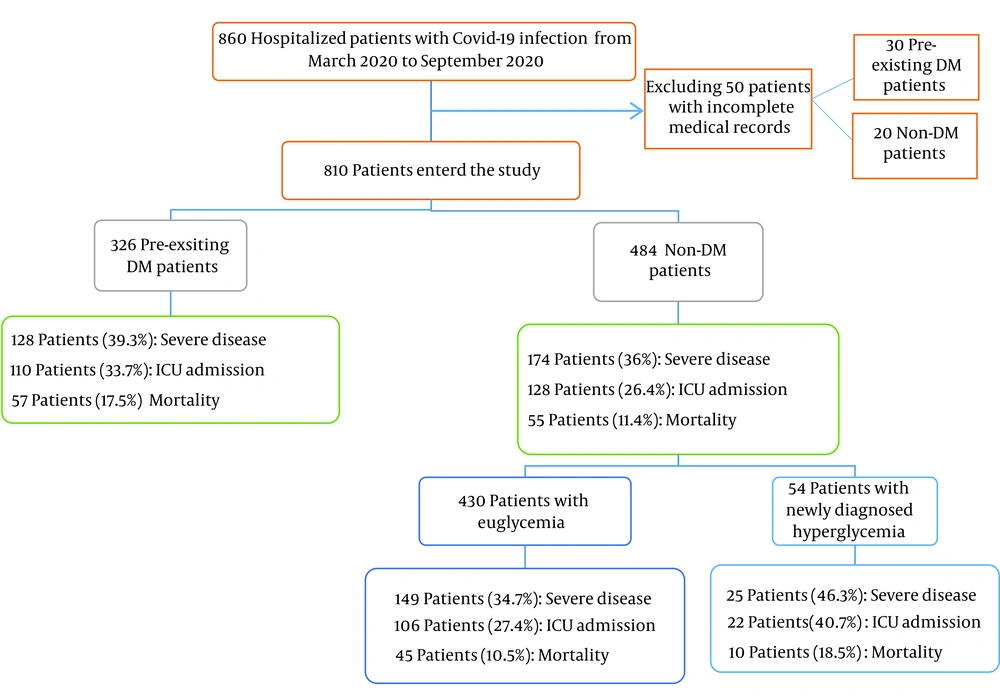
This study included 810 medical records of confirmed COVID-19 patients, of whom 326 had pre-existing DM, and 484 were non-DM (Figure 1). The mean age of the study subjects was 56 years, and 53.8% of them were male.
The mean age in the diabetic and non-diabetic groups was 61.3 years and 52.3 years, respectively, and patients with DM were older than their non-DM counterparts (P < 0.001).
Pre-existing DM and newly diagnosed hyperglycemia frequency rates were 40.2% and 11.2%, respectively. There was a significant difference in the mean HbA1C between newly diagnosed hyperglycemia (5.2% ± 0.8) and pre-existing DM (7.5% ± 1.5) (P-value < 0.0001). The mean duration of diabetes was 8.5 ± 6.5 years in patients with pre-existing DM (min: 1 year, max: 20 years). The rates of underlying diseases, including hypertension (35.3%), ischemic heart disease (17.9%), and chronic kidney disease (11.9%), were higher in diabetic patients than in non-diabetic and newly diagnosed hyperglycemia patients (P < 0.001) (Table 1). Although the rates of clinical features were not different between diabetic and non-diabetic patients, the rates of dyspnea and hemoptysis were higher in DM patients than in their non-DM and newly diagnosed hyperglycemia counterparts (Table 1).
| Characteristics | Non-DM (N = 430) a | Newly Diagnosed Hyperglycemia (N = 54) a | Pre-existing DM (N = 326) a | P-Value |
|---|---|---|---|---|
| Age (mean ± SD) | 51.8 ± 17.8 | 58.1 ± 15.7 | 61.2 ± 12.5 | < 0.0001 |
| Sex (male) | 253 (58.8) | 27 (50.0) | 156 (47.9) | 0.009 |
| Underlying diseases | ||||
| Hypertension | 98 (34.3) | 14 (4.9) | 178 (60.8) | < 0.0001 |
| Ischemic heart disease | 59 (34.1) | 16 (9.2) | 98 (56.6) | < 0.0001 |
| Heart failure | 15 (48.4) | 4 (12.9) | 12 (38.7) | 0.362 |
| Cerebrovascular disease | 8 (42.1) | 1 (5.3) | 10 (52.6) | 0.538 |
| Chronic kidney disease | 28 (29.2) | 7 (7.3) | 61 (63.5) | < 0.0001 |
| Lung Disease | 35 (76.1) | 2 (4.3) | 9 (19.6) | 0.005 |
| Liver disease | 1 (25) | 0 | 3 (75) | 0.355 |
| Clinical features | ||||
| Fever | 192 (56) | 19 (5.5) | 132 (38.5) | 0.282 |
| Dyspnea | 296 (55.2) | 38 (7.1) | 202 (37.7) | 0.113 |
| Cough | 251 (54.7) | 27 (5.9) | 181 (39.4) | 0.436 |
| Hemoptysis | 0 | 0 | 5 | 0.024 |
| Myalgia | 252 (55.1) | 22 (4.8) | 183 (40) | 0.044 |
| Loss of appetite | 121 (53.8) | 9 (4) | 95 (42.2) | 0.161 |
| Headache | 121 (53.1) | 9 (4) | 95 (42.2) | 0.856 |
| Diabetic ketoacidosis | 0 | 0 | 7 | 0.05 |
| Acute kidney injury | 77 (39.9) | 16 (8.3) | 100 (51.8) | < 0.0001 |
a Values are presented as No. (%) or mean ± SD.
Diabetic ketoacidosis was observed in 9 (1.1%) patients, all with pre-existing diabetes and 4 with severe COVID-19 (1.3%).
The rate of acute kidney injury was 23.8% (193 subjects), which was significantly higher in DM than in non-DM patients (P < 0.0001) (Table 1).
Moreover, it was also higher in patients with severe COVID-19 (27.8% vs. 21.5%, P = 0.04) (Table 2).
| Characteristics | Severe COVID-19 (N = 302) a | Non-Severe COVID-19 Infection (N = 508) a | P-Value |
|---|---|---|---|
| Age (mean ± SD) | 57.7 ± 15.8 | 55 ± 16.4 | 0.018 |
| Sex (male) | 189 (43.3) | 247 (56.7) | 0.0001 |
| Underlying diseases | |||
| DM | 128 (42.4) | 198 (39) | 0.33 |
| HTN | 118 (39.1) | 168 (33.1) | 0.08 |
| IHD | 54 (17.9) | 91 (17.9) | 0.99 |
| HF | 11 (3.6) | 20 (3.9) | 0.83 |
| CVA | 9 (3) | 10 (2) | 0.35 |
| CKD | 46 (15.2) | 50 (9.8) | 0.022 |
| Lung disease | 21 (7) | 25 (4.9) | 0.22 |
| Liver disease | 2 (0.7) | 2 (0.4) | 0.63 |
| Clinical features | |||
| Fever | 179 (59.3) | 271 (53.3) | 0.10 |
| Dyspnea | 232 (76.8) | 304 (59.8) | < 0.0001 |
| Cough | 193 (63.9) | 266 (52.4) | 0.0001 |
| Hemoptysis | 1 (0.3) | 4 (0.8) | 0.65 |
| Myalgia | 185 (61.3) | 272 (53.5) | 0.032 |
| Anorexia | 87 (28.8) | 138 (27.2) | 0.61 |
| Headache | 47 (15.6) | 92 (18.1) | 0.35 |
| AKI | 84 (27.8) | 109 (21.5) | 0.04 |
Abbreviations: DM, diabetes mellitus; HTN, hypertension; IHD, ischemic heart disease; CKD, chronic kidney disease; AKI, acute kidney injury.
a Values are presented as No. (%) or mean ± SD.
The mean total hospitalization days in survivors were 6.77 ± 3.94 days, and the length of hospitalization was longer in diabetic patients than in non-diabetic patients (7.15 ± 4.13 vs. 6.53 ± 3.8, P = 0.045)
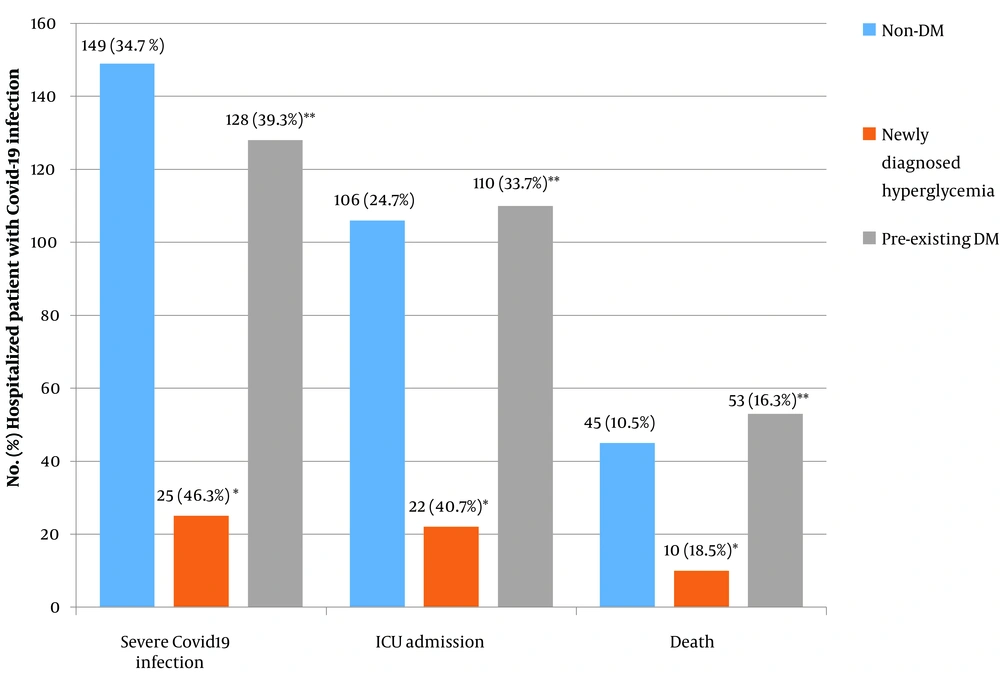
The rate of severe disease was higher in men, which increased with age. Pre-existing CKD was associated with the severity of COVID-19 (P = 0.022). The need for ICU admission and mortality rates were 29.4% and 13.3%, respectively. Diabetic patients had a greater need for ICU admission and death than non-diabetic patients (P = 0.025 and 0.044, respectively) (Figure 2).
The risk factors affecting death were age (OR: 1.030, 95% CI: 1.015 - 1.046, P < 0.0001), history of DM (OR = 1.51, 95% CI = 1.008 - 2.274, P = 0.044), history of CKD (OR: 2.10, 95% CI: 1.18 - 3.73, P = 0.011), and the incidence of AKI (OR: 2.93, 95% CI: 1.92 - 4.47, P < 0.0001). The risk factors affecting the need for ICU admission were age (OR: 1.541, 95% CI: 1.090 - 2.179 P = 0.014), sex (OR = 1.594, 95% CI: 1.168 - 2.176, P = 0.003), history of DM (OR = 1.41, 95% CI = 1.043 - 1.923, P = 0.025), history of CVD (OR = 1.568, 95% CI=1.053 - 2.334, P = 0.027), the incidence of newly diagnosed hyperglycemia (OR = 2.10, 95% CI = 1.170 - 3.774, P = 0.012), and the incidence of AKI (OR: 1.85, 95% CI: 1.260 - 2.273, P = 0.002) (Table 3).
| Variables | Mortality | ICU Admission | ||||
|---|---|---|---|---|---|---|
| P-Value a | OR | 95% CI | P-Value a | OR | 95% CI | |
| Age | < 0.0001 | 1.030 | 1.015 - 1.046 | 0.014 | 1.541 | 1.090 - 2.179 |
| Sex | 0.357 | 1.229 | 0.793 - 1.906 | 0.003 | 1.594 | 1.168 - 2.176 |
| DM | 0.044 | 1.510 | 1.008 - 2.274 | 0.025 | 1.41 | 1.043 - 1.923 |
| HTN | 0.122 | 1.421 | 0.911 - 2.217 | 0.375 | 1.163 | 0.833 - 1.625 |
| CVD | 0.482 | 1.206 | 0.716 - 2.033 | 0.027 | 1.568 | 1.053 - 2.334 |
| CKD | 0.011 | 2.10 | 1.18 - 3.73 | 0.506 | 1.177 | 0.728 - 1.903 |
| Newly diagnosed hyperglycemia | 0.297 | 0.673 | 0.319 - 1.417 | 0.012 | 2.10 | 1.170 - 3.774 |
| AKI | < 0.0001 | 2.930 | 1.920 - 4.470 | 0.002 | 1.85 | 1.260 - 2.273 |
Abbreviations: ICU, intensive care unit; DM: diabetes mellitus; HTN, hypertension; CVD, cardiovascular disease; CKD, chronic kidney disease; AKI, acute kidney injury; OR, odds ratio; CI, confidence interval.
a Statistically significant at P < 0.05
5. Discussion
According to the results of this cross-sectional study, the frequency of pre-existing DM among hospitalized patients with COVID-19 was 40.2%, higher than the global (9.3%), regional (12.2%), and Ahvaz City rates (15.1%) (12, 13).
This study observed newly diagnosed hyperglycemia in 54 (11.2%) patients, consistent with several studies. Farag et al., for example, reported that 13.5% (77/570) of their studied COVID-19 patients had no previous diagnosis of DM (6)
Sathish et al. conducted a meta-analysis of 8 studies with more than 3,700 patients and reported a pooled proportion of 14.4% for newly diagnosed diabetes in COVID-19 hospitalized patients (14).
According to the current study, the most common underlying diseases in patients with COVID-19 were HTN, IHD, and CKD, all being significantly more common among people with diabetes, which is in line with some previous studies.
Zhang et al. reported that 27% of COVID-19 patients had diabetes, 20% had IFG, and 53% had normal blood sugar. Also, underlying diseases, including HTN, IHD, and CKD, were more common in patients with diabetes (2).
Soliman et al. stated that the prevalence of diabetes in COVID-19 patients was 19.5%, and they were older and had a higher rate of underlying diseases than non-DM patients (15).
Guo et al. found that the most common underlying diseases among COVID-19 patients were DM and HTN. The rate of cardiovascular disease was also higher among people with diabetes than non-diabetics (16).
In Li et al.’s study, 38% of COVID-19 patients with DM were older than their counterparts who did not have DM (17).
In our study, the most common symptoms of COVID-19 on the first day of hospitalization were fever, dyspnea, cough, myalgia, loss of appetite, and headache. Hemoptysis was observed only in DM patients, while shortness of breath was significantly more common in non-DM patients, which is inconsistent with some previous studies. Zhang et al., for instance, found that the most common symptoms at hospital admission were fever, cough, lethargy, decreased appetite, dyspnea, and chest discomfort, and patients with diabetes were more likely to have dyspnea and loss of appetite than those without DM (18).
According to our results, DKA occurred in 2.8% of diabetics, but HHS was not observed. In Zhang et al., 2 (2%) of the patients developed DKA, and 1 (1%) developed drug-induced hypoglycemic coma, but no case of HHS was reported (2).
In our study, the rate of AKI was 23.8% in patients with COVID-19, which was higher in DM patients than in non-DM patients. Moreover, the rate of AKI was higher in severe COVID-19.
Chan et al. reported AKI in 46% of COVID-19 patients, and 19% of their patients required dialysis. Patients with AKI were older and more likely to have high blood pressure, diabetes, and congestive heart failure (19).
As known, AKI in COVID-19 patients is due to direct virus damage, unregulated inflammatory response, activation of the angiotensin 2 pathway, hyper-coagulation, and microangiopathy (19).
According to the results of the present study, 37.3% of the patients had severe disease at hospital admission based on the NEWS2 scoring system. Also, the severity of COVID-19 infection increased with age and was higher among men. Although the rate of severe disease was not different between diabetic and non-diabetic patients, the hospital stay length and ICU admission and death rates were higher in diabetic patients than in non-DM patients.
The patients with pre-existing CKD were more likely to have severe COVID-19. Therefore, the NEWS2 scoring system may not be appropriate for measuring COVID-19 severity in individuals with underlying diseases, and it may require modification and inclusion of other parameters, such as the history of underlying disease and drug use.
In a study by Soliman et al., diabetics had a higher rate of severe pneumonia and ARDS, ICU hospitalization, mechanical ventilation, and oxygen therapy (15).
Zhang et al. reported that patients with diabetes and impaired fasting glucose had more severe diseases than others. In addition, diabetes and IFG were associated with an increased mortality risk (2).
In Zhou et al.’s study, the risk of in-hospital death was higher in subjects with diabetes, cardiovascular disease, and hypertension than in others (20).
According to Alshukry et al., people with diabetes were likely to suffer from more severe COVID-19 and experience higher mortality and pre-existing hypertension than those without DM (21).
Li et al. found high hypertension and cerebrovascular disease prevalence in patients with severe disease and those admitted to the ICU. However, the prevalence of DM was not high in patients with severe disease (22). In contrast, Fadini et al. concluded that DM did not augment the risk of COVID-19 infection but could increase adverse outcomes of COVID-19 (23).
Guo et al. reported that the mortality rate was not higher among individuals with DM than among non-diabetic patients. However, when individuals with other comorbidities were excluded to eliminate the effect of other comorbidities, people with diabetes were older than non-diabetics, had more common symptoms of nausea and vomiting, and had a higher mortality rate (16).
5.1. Limitations
The limitations of this study were as follows:
1) This was a cross-sectional study based on patient medical records, and patients with newly diagnosed hyperglycemia were not followed up after recovery from COVID-19 infection.
2) This study included only hospitalized COVID-19 patients with moderate to severe conditions, so the results could not be generalized to all patients with COVID-19.
3) HbA1C was not measured in all COVID-19 patients. During the hospital stay, HbA1C was measured on the first day of admission of DM patients and those with two or more blood glucose tests of > 180 mg/dL.
4) The severity of COVID-19 infection was estimated based on the NEWS2 system on the first day of hospital admission, and this system may not be appropriate for COVID-19 infection in patients with underlying diseases.
5.2. Conclusions
Many hospitalized patients with COVID-19 infection had either pre-existing DM (40.2%) or newly diagnosed hyperglycemia (11.2%). Hypertension, IHD, and CKD were reported as the most common comorbidities in patients with COVID-19 infection, and they were significantly more common in COVID-19 patients with pre-existing DM than in their non-DM counterparts. Also, COVID-19 patients with newly diagnosed hyperglycemia had a higher rate of severe COVID-19, ICU hospitalization, and mortality than euglycemic patients. The rate of acute kidney injury was higher in DM patients than in non-DM ones and patients with severe COVID-19 infection. The results of this study suggest that the blood glucose levels of all patients with COVID-19 infection should be constantly checked for early diagnosis and effective treatment of newly diagnosed hyperglycemic patients.