1. Background
Cystic echinococcosis (CE) arises from infection by the dog tapeworm, Echinococcus granulosus, during its larval (metacestode) stages (1). In the life cycle of E. granulosus, dogs and canids serve as primary definitive hosts, while ungulates (such as sheep, goats, and pigs) and humans act as intermediate hosts. The adult cestode produces infective eggs through sexual reproduction in the small intestine of a definitive host. Intermediate hosts become infected through direct contact with a dog or indirectly via the ingestion of egg-contaminated water, soil, or vegetables (2). Following infection, the intermediate host typically develops E. granulosus cysts primarily in the liver (70%), lung (20 - 30%), and less frequently in the kidney, spleen, brain, heart, and other organs (3, 4). Cystic echinococcosis is classified as one of the 17 neglected tropical diseases, prevalent in various parts of the world, particularly in rural areas, and endemic in regions including South America, North and East Africa, Asia, Australia, and China (5, 6).
Currently, surgical removal of CE cysts remains the primary and preferred treatment method (7). Besides surgery, alternative treatment options for CE include chemotherapy with benzimidazoles, PAIR (puncture-aspiration-injection-reaspiration), and a watch/wait approach (8). However, during surgery, the leakage of protoscoleces-rich fluid into adjacent tissues poses a significant risk of secondary echinococcosis and recurrence (9, 10). Hence, the use of effective scolicidal agents is crucial to prevent such complications (11). Various scolicidal agents like chlorhexidine gluconate, hypertonic saline, silver nitrate, cetrimide, ethyl alcohol, and povidone-iodine are currently employed for protoscoleces inactivation during surgery (8). However, many of these agents are associated with different complications, including sclerosant cholangitis, liver necrosis, and methemoglobinemia. Therefore, the development of new scolicidal agents with fewer side effects, lower cost, and higher efficiency is imperative (12, 13).
Due to the reported side effects of biochemical agents and the quest for safer and more effective treatments, current medical research has increasingly focused on traditional and herbal medicine. Plant extracts are widely used in developing countries due to their availability, safety, and cost efficiency. Investigating the scolicidal effects of herbal extracts like Urtica dioica and Pyrus boissieriana presents a promising avenue for developing alternative treatments for CE (14).
Urtica dioica, commonly known as the nettle plant, is a perennial flowering plant cultivated in temperate regions and belonging to the family Urticaceae (15, 16). Various studies have demonstrated its diverse medicinal properties, including antioxidant, antiulcer, anticancer, antiviral, antibacterial, antifungal, and immunomodulatory activities. Urtica dioica has been noted for its potential in managing hypertension and hyperglycemia, with its root commonly used in the treatment of prostatic hyperplasia. Additionally, its leaf extract has been traditionally utilized to alleviate pain and inflammation, making it a folk remedy for conditions such as arthritis (17). Carvacrol, a major compound found in U. dioica, exhibits significant antiparasitic and antifungal properties (18).
Pyrus boissieriana, known as the wild pear, belongs to the Rosaceae family and is predominantly found in the northern regions of Iran, particularly the Hyrcanian forests (19). Studies have highlighted its notable antioxidant activities and its effectiveness in inhibiting α-glucosidase, suggesting its potential as an antidiabetic agent (20). Pyrus boissieriana also demonstrates antihyperlipidemic and diuretic effects, with its leaves traditionally used to address hypertension, bladder inflammation, bacteriuria, and renal stones. Arbutin, a hydroquinone derivative abundant in P. boissieriana, possesses antioxidant, antibacterial, and antifungal properties, along with skin whitening and UV-protective effects (21).
2. Objectives
Given the beneficial properties of U. dioica and P. boissieriana and the imperative to explore novel therapeutic approaches for CE, endemic in the region, we investigated the in vitro scolicidal effects of their extracts on protoscoleces of CE cysts.
3. Methods
3.1. Collection and Identification of the Plants
Pyrus boissieriana and U. dioica were collected during the summer of 2019 from Guilan Province, Northern Iran, and identified by professors of Pharmacognosy at the Faculty of Pharmacy of Shiraz University of Medical Sciences. Subsequently, the aerial parts of P. boissieriana, including flowers, and the leaves of U. dioica were separated and air-dried under shade.
3.2. Preparation of Hydroalcoholic Extract of Urtica dioica and Chloroformic Extract of Pyrus boissieriana
For the hydroalcoholic extract of U. dioica, the dried leaves underwent a soaking method. Initially, 150 g of dried leaves were combined with 300 ml of a hydroalcoholic solvent (70% methanol and 30% water) in an Erlenmeyer flask. Covered with aluminum foil, the flask was shaken overnight. Subsequently, the solution was filtered and refrigerated at 4°C. Using a rotary evaporator, the methanol extracts were completely evaporated. The remaining water portion was subjected to dry ice to form a thick extract, which was then stored in amber bottles at 4°C for future use.
To obtain the chloroformic extract of P. boissieriana, 250 g of dried plant material was powdered using an electric blender. The powder was extracted via the percolation method using a hydroalcoholic solution at a ratio of 4:1. The resulting solution was decanted with chloroform at a ratio of 1:10, with each hydroalcoholic extract washed three times with chloroform. After separation of the chloroform phase, the solution was evaporated using a rotary evaporator and dried to obtain the powder for further use (13).
3.3. Obtaining and Preparation of Protoscoleces
Cystic echinococcosis cyst samples were collected from slaughtered sheep at slaughterhouses under sterile conditions and promptly transported to the Parasitology Laboratory at the Department of Parasitology and Mycology, affiliated with Shiraz University of Medical Science. Cysts with a single cavity containing sufficient protoscoleces were selected. Upon arrival at the laboratory, the cyst surfaces were washed with 70% alcohol in a sterile environment. The CE fluid containing protoscoleces was aspirated using a sterile 50 mL syringe and transferred to Falcon tubes. After centrifugation for two minutes at 1500 rpm to sediment the protoscoleces, the supernatant was transferred to another container. The collected protoscoleces were washed three times with normal saline, and isolated protoscoleces were obtained from the cyst-infected livers (22).
3.4. Effectiveness of Urtica dioica and Pyrus boissieriana Extracts on Protoscoleces
To assess the scolicidal activity of the hydroalcoholic extract of U. dioica and the chloroformic extract of P. boissieriana on protoscoleces of CE cysts, five concentrations (5, 10, 20, 40, and 80 mg/ml) of the extracts were applied for durations of 10, 20, 30, 60, and 120 minutes. Initially, a suspension of protoscoleces (3 × 103 washed protoscoleces) was placed in test tubes, and each concentration was added to the respective tube, gently mixed, and then incubated at 37°C for the specified durations. Subsequently, the upper phase of the mixture was carefully removed, and 0.1% eosin stain was added to the pellet of protoscoleces, which was then gently mixed. Protoscoleces sediment was smeared onto glass slides, and the viability of protoscoleces was determined (23). Sterile normal saline containing tween 20 served as the negative control group, while hypertonic saline (20%) served as the positive control group. All experiments were conducted in triplicate across 48 plates, and the mean and standard deviation (SD) were calculated for each sample.
3.5. Viability Test
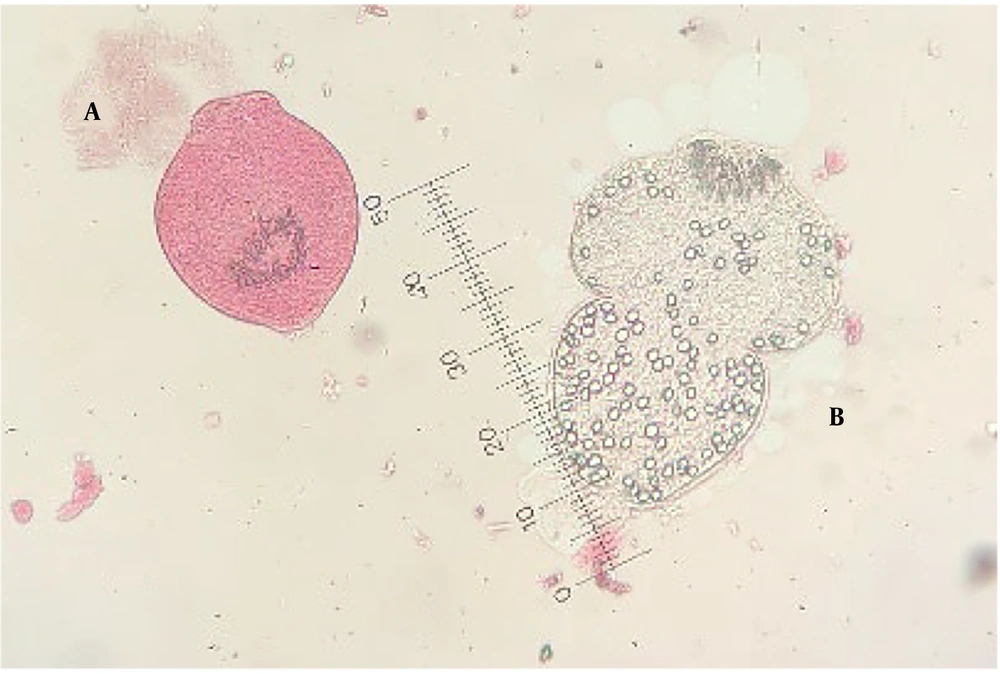
To assess protoscoleces viability, 0.1% eosin staining (1 gram of eosin powder in 1000 mL distilled water) was employed. Following staining, protoscoleces were evaluated for flame cell motility under a light microscope and impermeability to 0.1% eosin solution. Living protoscoleces remained colorless and transparent, whereas dead protoscoleces were stained red (Figure 1).
3.6. Statistical Analysis
Statistical analysis was conducted using GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, California, USA). Differences in scolicidal effects among various concentrations of U. dioica, P. boissieriana, and control groups were analyzed using one-way analysis of variance (ANOVA) followed by the Games-Howell multiple comparisons test (24). Additionally, t-tests were employed to assess differences in scolicidal effects between U. dioica and P. boissieriana at each concentration and exposure time. Results were reported as mean ± SD, with P-values < 0.05 considered statistically significant.
4. Results
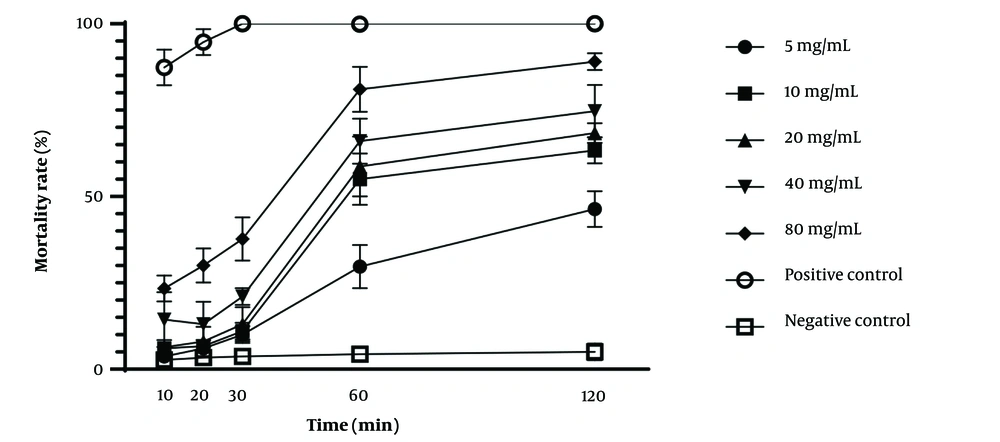
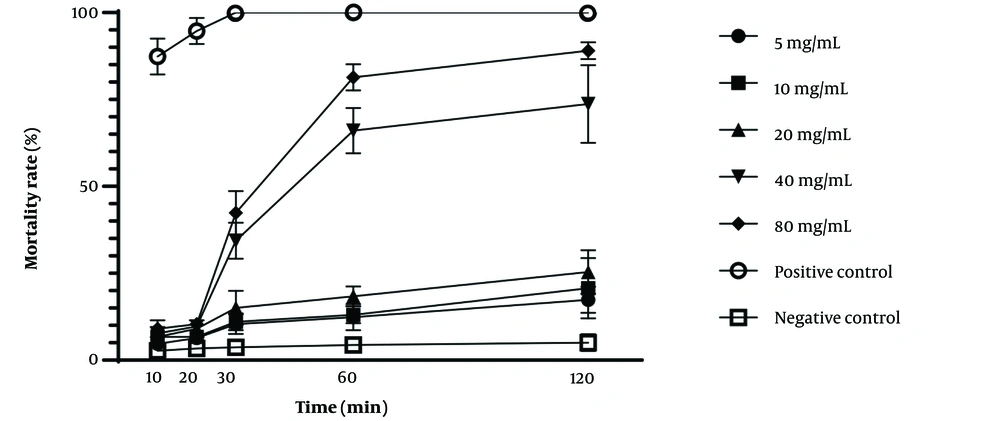
The protoscolicidal activity of U. dioica and P. boissieriana extracts at various concentrations following different exposure times is demonstrated in Table 1. Additionally, the scolicidal effectiveness of the studied extracts is illustrated in Figures 2, and 3, considering time and concentration. The scolicidal effects of U. dioica extract at a concentration of 80 mg/mL were 81% and 89% after 60 and 120 minutes of exposure, respectively. Similarly, almost identical results were obtained for P. boissieriana extract at a concentration of 80 mg/mL (81.33% and 89% after 60 and 120 minutes of exposure, respectively), which significantly differed from the negative control group (P < 0.001). However, U. dioica extract exhibited stronger scolicidal effects compared to P. boissieriana extract. Specifically, the scolicidal activity of U. dioica was significantly higher than that of P. boissieriana at concentrations lower than 40 mg/mL after 60 and 120 minutes of exposure, and at a concentration of 80 mg/mL after 10 and 20 minutes of exposure (P < 0.001). ANOVA analysis revealed that the protoscolicidal activity of P. boissieriana extract was significantly higher at concentrations of 40 and 80 mg/mL compared to lower concentrations after 30, 60, and 120 minutes of exposure (P < 0.001). The lowest protoscolicidal activity was observed in U. dioica at a concentration of 5 mg/mL after 10 minutes of exposure (3.67%). Our study indicates that both extracts exhibit dose-dependent and time-dependent protoscolicidal activity.
The relationship between the in vitro mortality rate of protoscoleces of Echinococcus granulosus and different concentrations of the hydroalcoholic extract of Urtica dioica following various exposure times, compared to negative and positive controls. Each point represents the mean percentage of dead protoscoleces from three different experiments.
The relationship between the in vitro mortality rate of protoscoleces of Echinococcus granulosus and different concentrations of the chloroform extract of Pyrus boissieriana following various exposure times, compared to negative and positive controls. Each point represents the mean percentage of dead protoscoleces from three different experiments.
| Exposure Time, min and Concentrations of the Extracts, mg/mL | Urtica dioica Extract | Pyrus boissieriana Extract | P-Values b of the Comparison Between U. dioica & P. boissieriana Extracts c | ||
|---|---|---|---|---|---|
| Means of Mortality % | P-Value a | Means of Mortality % | P-Value a | ||
| 10 | |||||
| 5 | 3.67 ± 0.58d | < 0.001 | 4.67 ± 0.58 d, e | < 0.001 | 0.1 |
| 10 | 6.00 ± 1.00 d, e | 6.67 ± 1.16 d, a | 0.49 | ||
| 20 | 6.33 ± 0.58 d, e | 6.67 ± 0.58e, a | 0.51 | ||
| 40 | 14.33 ± 3.22 e, a | 7.67 ± 0.58 a | 0.06 | ||
| 80 | 23.33 ± 1.53 a | 9.00 ± 1.00 a | < 0.001 | ||
| Positive control | 87.33 ± 2.08 | 87.33 ± 2.08 | - | ||
| Negative control | 2.67 ± 0.58d | 2.67 ± 0.58 d | - | ||
| 20 | |||||
| 5 | 6.00 ± 1.00 d, c | < 0.001 | 6.33 ± 0.58 d | < 0.001 | 0.64 |
| 10 | 6.67 ± 0.58 d, e | 6.67 ± 0.58 d | 0.99 | ||
| 20 | 8.00 ± 1.73 d, e, a | 9.00 ± 1.00 d, e | 0.43 | ||
| 40 | 13.00 ± 2.65 d, e, a | 9.67 ± 0.58 e | 0.1 | ||
| 80 | 30.00 ± 2.00 | 10.33 ± 0.58e | < 0.001 | ||
| Positive control | 94.67 ± 1.53 | 94.67 ± 1.53 | - | ||
| Negative control | 3.33 ± 0.58 c, a | 3.33 ± 0.58 | - | ||
| 30 | |||||
| 5 | 10.00 ± 1.00 d | < 0.001 | 10.33 ± 1.16 d | < 0.001 | 0.72 |
| 10 | 11.00 ± 1.00 d | 11.00 ± 1.00 d | 0.99 | ||
| 20 | 13.00 ± 2.00 d | 15.00 ± 2.00 d | 0.28 | ||
| 40 | 21.00 ± 1.00 | 34.33 ± 2.08e | 0.001 | ||
| 80 | 37.67 ± 2.52 | 42.33 ± 2.52 e | 0.08 | ||
| Positive control | 100.00 ± 0.00 | 100.00 ± 0.00 | - | ||
| Negative control | 3.67 ± 0.58 | 3.67 ± 0.58 | - | ||
| 60 | |||||
| 5 | 29.67 ± 2.52 | < 0.001 | 12.33 ± 1.53 a | < 0.001 | 0.001 |
| 10 | 55.00 ± 3.00 d | 13.00 ± 1.00d | < 0.001 | ||
| 20 | 58.67 ± 3.51 d | 18.33 ± 1.16 | < 0.001 | ||
| 40 | 66.00 ± 2.65 a | 66.00 ± 2.65 | 0.99 | ||
| 80 | 81.00 ± 2.65 | 81.33 ± 1.53 | 0.85 | ||
| Positive control | 100.00 ± 0.00 | 100.00 ± 0.00 | - | ||
| Negative control | 4.33 ± 0.58 | 4.33 ± 0.58 | - | ||
| 120 | |||||
| 5 | 46.33 ± 2.08 | < 0.001 | 17.33 ± 1.53 d | < 0.001 | < 0.001 |
| 10 | 63.33 ± 1.53 d | 20.67 ± 3.51d, e, a | < 0.001 | ||
| 20 | 68.33 ± 1.16d | 25.33 ± 2.52 a | < 0.001 | ||
| 40 | 74.67 ± 3.06d | 73.67 ± 4.51 b | 0.76 | ||
| 80 | 89.00 ± 1.00 | 89.00 ± 1.00b | 0.99 | ||
| Positive control | 100.00 ± 0.00 | 100.00 ± 0.00 | - | ||
| Negative control | 5.00 ± 1.00 | 5.00 ± 1.00e | - | ||
Protoscolicidal Effects of Different Concentrations of Urtica dioica and Pyrus boissieriana on 3 × 103 Protoscoleces of Cystic Echinococcosis Cyst after 10, 20, 30, 60, and 120 Minutes
5. Discussion
The results of our study demonstrate that the extracts of U. dioica and P. boissieriana exhibit strong scolicidal activity, particularly at higher concentrations. While various studies have explored the protoscolicidal effects of hypertonic saline (25), cetrimide, ethyl alcohol (95%) (26), silver nitrate (27), 10% povidone-iodine (28), albendazole (29), and mannitol (27), their usage is limited due to multiple dangerous complications (3). Consequently, there is an urgent need for novel therapeutic approaches due to the inadequate therapeutic effects of existing drugs. Natural remedies and herbal products have long been utilized in treating microbial and fungal infections (30), owing to their biodegradability and safe nature for host organs. In recent decades (31), traditional and natural medicines have undergone detailed study for drug discovery purposes. Additionally, natural and herbal products serve as inspiration for developing new medications to treat various diseases (32). Although the extract of P. boissieriana exhibited lower potency compared to U. dioica at lower concentrations, both extracts at a concentration of 80 mg/mL showed significant scolicidal effects after 60 and 120 minutes. Therefore, these natural extracts have the potential to be utilized as safe medications in the treatment of CE.
Numerous studies have explored the effects of various herbal extracts on the protoscoleces of CE cysts. In 2008, Sadjjadi et al. conducted a study assessing the in vitro effects of different Allium sativum extracts on protoscoleces. They found that the chloroform extract of garlic at a concentration of 200 mg/mL exhibited the highest scolicidal activity, with a mortality rate of 99.97% and 100% after 60 and 120 minutes of exposure, respectively (33). Conversely, the aqueous extract of garlic at a concentration of 500 mg/mL showed the lowest scolicidal activity, with mortality rates of 29.51% and 40.6% after 60 and 120 minutes of exposure, respectively. A study conducted in 2019 investigated the protoscolicidal effects of the methanolic extract of Allium hirtifolium on protoscoleces of E. granulosus. It revealed that at a concentration of 50 mg/mL of A. hirtifolium extract, only a 16.96% scolicidal effect was observed after 50 minutes of exposure. Despite belonging to the allium family, A. hirtifolium's methanolic extract demonstrated less scolicidal effect (23). Another study by Zibaei et al. in 2012 evaluated the scolicidal effects of Satureja khuzestanica and Olea europaea against CE, showing S. khuzestanica to be a more effective scolicidal agent. At 0.01% concentrations, S. khuzestanica exhibited 83.3% and 68.6% scolicidal effects after 60 and 120 minutes of exposure, respectively (34), which aligns closely with our results. U. dioica and P. boissieriana, at a concentration of 80 mg/mL, demonstrated approximately 81% and 89% mortality rates, respectively, after 60 and 120 minutes of exposure, consistent with these findings. Moazeni and Roozitalab investigated the scolicidal effects of Zataria multiflora on protoscoleces and found that the extract of Z. multiflora is a potent protoscolicidal agent (35).
Urtica dioica has been utilized for centuries in treating various ailments. Previous studies have confirmed its antioxidant, antiviral, antibacterial, antifungal, anthelmintic, and analgesic properties, alongside its richness in minerals and vitamins (20, 36). Hadizadeh et al. explored the fungicidal potential of U. dioica against plant pathogenic fungi, establishing its efficacy as a strong fungicide (37). Mikaeili et al. investigated its antifungal effects against Microsporum canis both in vitro and in a guinea pig model, suggesting its topical application for treating tinea corporis (38). Sarma Kataki demonstrated the dose-dependent anthelmintic activity of the methanolic extract of U. dioica against adult Indian earthworms (Pheretima posthuma) (39). Similarly, Turel et al. examined its anthelmintic potency against mice naturally infected with Aspiculuris tetraptera, confirming its effectiveness (40). A 2020 study validated the dose-dependent anti-leishmania effects of U. dioica in vitro and in vivo (41). Additionally, Yongabi et al. evaluated its activity against multiple fungi, affirming its potent antifungal properties without significant side effects, attributed to the plant lectin agglutinin and its effective component (42), carvacrol (18). Furthermore, tannin, another compound found in U. dioica, has been shown to inhibit enzymes and prevent microbial adhesion, rendering the plant effective against bacteria and fungi (43). Given the favorable outcomes of our study and other research, U. dioica could serve as a safe protoscolicidal agent.
Several studies have highlighted the antibacterial, antifungal, antioxidant, and antidiabetic activities of P. boissieriana (21). Azadbakht et al. demonstrated its antilarval, antibacterial, and antifungal properties against Candida albicans and Cladosporium. Arbutin, a significant component of P. boissieriana, exhibited antifungal and antioxidant effects (44). Additionally, arbutin was found to possess significant antibacterial activity, particularly in treating urinary tract infections (45). Jin and Sato investigated the antibacterial effects of aqueous extracts from young shoots of Pyrus spp., identifying benzoquinone, derived from arbutin, as a potent antibacterial agent (46). Güven et al. confirmed significant antifungal effects of Pyrus serikensis (47), while a recent study in 2019 established the antifungal effects of Pyrus communis against Aspergillus flavus and Candida albicans, along with antibacterial activities against Salmonellatyphimurium (48). Considering this evidence alongside our study results, P. boissieriana shows promise in treating CE.
Natural remedies are widely used for their safety and minimal side effects in treating infections globally (31, 49-51). However, this study did not evaluate the cytotoxic effects of the extracts, which is a limitation. Nonetheless, other studies have assessed and confirmed the safety of these extracts. Asadi and Abbasi Maleki demonstrated the LD50 of hydroalcoholic extract U. dioica in mice as 2900 mg/kg during acute toxicity testing, much higher than the doses used in our experiment (52). Similarly, P. boissieriana extract given to mice models at doses of 500 and 1000 mg/kg showed no reported side effects or mortalities (21, 53). Other Pyrus species, such as Pyrus pashia and P. communis, exhibited LD50 of 1500 and 2000 mg/kg, respectively, in rat models (54, 55), further supporting the safety of these herbal extracts in in vivo models.
5.1. Conclusions
In conclusion, this in vitro study suggests that both U. dioica and P. boissieriana extracts possess significant protoscolicidal activities and could serve as alternative natural medicines for treating CE. Furthermore, these plants and their properties could inspire the development of new scolicidal agents. However, further investigations are necessary to identify and isolate the active components of these extracts and to explore their therapeutic effects in in vivo models. Despite the absence of significant side effects in animals and humans thus far, additional studies are warranted in this regard.