1. Background
Ultraviolet (UV) radiation from compact fluorescent lamps (CFLs) may have both favorable and unfavorable effects on biological organisms. The beneficial effects of UV radiation are production of vitamin D and also the treatment of vitiligo, psoriasis, eczema, and jaundice (1-4). Nevertheless, it also has a lot of harmful effects such as apoptosis induction, DNA injury, suppression of immune system, cellular aging, oxidative damage, inflammation, elevation of liver enzymes, damage of human eye, and tumor formation (5-8). The damage of radiation can be due to free radical production (9). UV radiation has a potent effect on biological organisms (10). Bone marrow and lymphoid nodes are mostly affected by UV radiation. They produce leukocytes and their subtypes with an important role in the human immune system (11). The bone marrow also produces red blood cells (RBCs). Platelets are also produced by bone marrow, which have a role in hemostasis (12).
Recent studies have shown that UV exposure induces both local and systemic immune suppression and decreases induction of regulatory T cells (13). In different systems, the ability of cells to recognize antigen and also the stimulation of T cells is stopped by UV radiation (14). Furthermore, UV radiation changes the expression of the major histocompatibility complex (MHC) with a vital role in the immune system (15). Another study has shown that UV radiation causes apoptosis of B lymphocytes, resulting decrease in immunoglobulins such as IgM, IgG, IgA, and IgE (16).
Curcumin is a natural antioxidant that interferes with free radicals before they can damage the body (9). Curcumin explores free radicals and prevents excessive fat oxidation and oxidative DNA damage (17). In recent decades, antioxidant therapy has been widely studied for prevention of many diseases. Having an antioxidant effect, curcumin has also been used for this purpose (18). It is a yellow compound derived from the curcuma longa plant, which is native to Southeast Asia and has been used in traditional medicine for centuries (19). The main component of turmeric is curcumin (20). The yellow pigment of curcumin has a wide range of biological and pharmacological activities (21).
2. Objectives
There are only a few studies regarding the antioxidative effects of curcumin on hematological complications of CFLs. As many studies have shown the oxidative effects of ultraviolet rays, we aimed to assess the curcumin effects on the complications of CFLs on leukocyte and platelet counts and the morphology of leukocytes.
3. Methods
3.1. Animals
We carried out the study on 48 young adult (3 - 4 months old) male Sprague-Dawley rats (230 ± 30 g body weight) in March 2016. All of the animals were standard and they were kept in standard conditions including 22 ± 2°C, ad libitum feeding and 12-hour light/12-hour dark cycle.
3.2. Treatment Groups
The animals were randomly divided into the following six groups (n = 8) using simple randomization: group I: received ethyl oleate (curcumin solvent) 0.2 mL, IP, for 10 days without exposure to CFL; group II: received ethyl oleate 0.2 mL, IP, for 30 days, without CFL exposure; group III: daily 12-hours exposure to CFLs and injection of ethyl oleate 0.2 mL, IP, for 10 days; group IV: daily 12- hours exposure to CFLs and injection of ethyl oleate 0.2 mL, IP, for 30 days; group V: pre-treatment with curcumin (20 µmol, IP) in addition to 12- hours exposure to CFLs for 10 days; group VI: pre-treatment with curcumin (20 µmol, IP) in addition to 12- hours exposure to CFLs for 30 days.
3.3. Reagents and Materials
We used four compact fluorescent lamps (40 watts) emitting essentially at 280 - 400 nanometers (ROHS, China). After warming up for 10 minutes, we measured the UV emission of the lamps using the spectrofluorometer model UV-340-A. Curcumin and ethyl oleate were obtained from Sigma-Aldrich. The curcumin (100 mg) was dissolved in 10 mL of ethyl oleate. The animals were kept in aluminum-covered 1 × 1 × 1 meter boxes. We put the lamps at a distance of 10 cm from the animals. The lamps had the intensity of (UVA 1.06W/m2) and (UVB 0.02 Wm2). Twenty–four hours after the last UV exposure, we anesthetized the rats using pentobarbital (40 mg/kg, IP). Then, we collected samples of blood from the right atriums in an anticoagulant test tube containing 0.5 M EDTA and glass tubes without any anticoagulants. The total number of leukocytes and platelets were determined by the animal cell counter (model MS9+5, Romania). For the morphological study of leukocytes, drops of blood samples were put on slides and then, blood smears were obtained. The blood smears were stained using Wright-Giemsa.
3.4. Statistical Analysis
Data are given as mean ± standard deviation (SD) for each group. Statistical significance of differences was assessed with one-way analysis of variance (ANOVA) with Tukey’s post hoc test. We considered P < 0.05 as significant. All analyses were conducted using IBM SPSS Statistics 22 (SPSS Inc., Chicago, IL, USA).
3.5. Ethical Approval
Experiments were reviewed by the Ethics Committee, Urmia University of Medical Sciences, Urmia, Iran and approved with the decision number 1394-0-32-1734 in June 2015. Experiments were according to the guidelines of 1975 Declaration of Helsinki. In addition, all of the procedures on rats were carried out due to the “Principles of Laboratory Animal Care” (NIH publication No. 85-23, revised 1985).
4. Results
Hematological and morphological findings have been demonstrated in Table 1 and Figures 1 - 5. As shown in Table 1, the number of leukocytes was significantly lower in group III (8.36 ± 0.23 × 103/µL) and group IV (7.85 ± 0.18 × 103/µL) in comparison with group I (8.78 ± 0.14 × 103/µL) and group II (8.50 ± 0.20 × 103/µL) (P value III:I = 0.004, and P value IV:II < 0.0001). In case of using curcumin following CFL exposure, the levels of leukocytes were reached to the normal levels both at 10 and 30 days in group V (8.85 ± 0.29 × 103/µL) and group VI (8.30 ± 0.16 × 103/µL); there were no significant differences after 10 and 30 days between control and curcumin groups (P value V:I = 0.79, and P value VI:II = 0.09).
| Group I | Group III | Group V | Group II | Group IV | Group VI | P Value III:I | P Value V:I | P Value IV:II | P Value VI:II | P Value V:III | P Value VI:IV | P Value IV:III | P Value VI:V | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC × 103/µL | 8.78 ± 0.14 | 8.36 ± 0.23 | 8.85 ± 0.29 | 8.50 ± 0.20 | 7.85 ± 0.18 | 8.30 ± 0.16 | 0.004* | 0.790 | < 0.0001* | 0.090 | 0.001* | < 0.0001* | < 0.0001* | < 0.0001* |
| N × 103/µL | 1.71 ± 8.32 | 1.84 ± 30.32 | 1.73 ± 14.06 | 1.91 ± 9.83 | 2.24 ± 15.76 | 2.04 ± 55.13 | < 0.0001* | 0.307 | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* |
| E × 103/µL | 0.09 ± 1.20 | 0.02 ± 1.31 | 0.02 ± 0.76 | 0.09 ± 1.51 | 0.04 ± 1.31 | 0.02 ± 0.76 | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* | 0.195 | < 0.0001* | < 0.0001* | 0.482 |
| M × 103/µL | 0.09 ± 1.20 | 0.05 ± 33.70 | 0.09 ± 1.31 | 0.02 ± 0.76 | 0.04 ± 1.31 | 0.02 ± 0.76 | 0.004* | >0.99 | < 0.0001* | >0.99 | 0.004* | < 0.0001* | 0.435 | < 0.0001* |
| L × 103/µL | 6.89 ± 6.05 | 6.45 ± 62.69 | 7.01 ± 42.31 | 6.48 ± 4.54 | 5.53 ± 38.03 | 6.21 ± 55.17 | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* | < 0.0001* |
| PLTt × 103/µL | 851 ± 3.06 | 848 ± 4.50 | 846 ± 5.60 | 846 ± 2.73 | 750 ± 6.52 | 808 ± 2.13 | 0.243 | 0.053 | < 0.0001* | < 0.0001* | 0.688 | < 0.0001* | < 0.0001* | < 0.0001* |
Leukocyte and Platelet Counts After 10 and 30 Days in Control, Fluorescent, and Curcumin Groups and Their Statistical Comparisona
The subtypes of leukocytes including neutrophil, eosinophil, monocyte, and lymphocyte were changed by exposure to CFL (Table 1).
The number of neutrophils was significantly higher in group III (1.84 ± 30.32 × 103/µL) and group IV (2.48 ± 15.76 × 103/µL) compared to group I (1.71 ± 8.32 × 103/µL) and II (1.91 ± 9.83 × 103/µL) (P value III:I < 0.0001, and P value IV:II < 0.0001). In case of administration of curcumin following CFL exposure, the level of neutrophils was reached to a normal level in 10 days in group V (1.73 ± 14.06 × 103/µL) (P value V:I = 0.31); however, it was not reached to a normal level in 30 days in group VI (2.04 ± 55.13 × 103/µL) (P value VI:II < 0.0001). There were not significant differences after 10 days between the control and curcumin groups (P value V:I = 0.307). However, there was a significant difference after 30 days between the two groups (P value VI:II < 0.0001).
In addition, the number of eosinophils was significantly lower in groups III (0.02 ± 1.31 × 103/µL), IV (0.04 ± 1.31 × 103/µL), V (0.02 ± 0.76 × 103/µL), and VI (0.02 ± 0.76 × 103/µL) in comparison with groups I (0.09 ± 1.20 × 103/µL) and II (0.09 ± 1.51 × 103/µL) (P value III:I < 0.0001, P value IV:II < 0.0001, P value V:I < 0.0001, and P value VI:II < 0.0001).
The number of monocytes was significantly lower in group III (0.05 ± 33.70 × 103/µL) compared to group I (0.09 ± 1.20 × 103/µL) (P value III:I = 0.004). Whereas, the number of monocytes was significantly higher in group IV (0.04 ± 1.31 × 103/µL) in comparison with group II (0.02 ± 0.76 × 103/µL) (P value III:I < 0.0001). After application of curcumin following CFL exposure, the level of monocytes was reached to a normal level both after 10 and 30 days in groups V (0.09 ± 1.31 × 103/µL) and VI (0.02 ± 0.76 × 103/µL) (P value V:I > 0.99 and P value VI:II > 0.99). Thus, there were no significant differences after 10 and 30 days between the control and curcumin groups.
The number of lymphocytes was significantly lower in groups III (6.45 ± 62.69 × 103/µL) and IV (5.53 ± 38.03 × 103/µL) compared to the groups I (6.89 ± 6.05 × 103/µL) and II (6.48 ± 4.54 × 103/µL) (P value III:I < 0.0001 and P value IV:II < 0.0001). After usage of curcumin following CFL exposure, the level of lymphocytes was increased to a normal level after 10 days in group V (7.01 ± 42.31 × 103/µL) (P value V:III < 0.0001). The number of lymphocytes was considerably lower in group V compared to group I, however, it is reached near the normal level after 30 days in group VI (6.21 ± 55.17 × 103/µL) (P value VI:II < 0.0001).
The number of platelets (PLTs) was significantly lower in group IV (750 ± 6.52 × 103/µL) and VI (808 ± 2.13 × 103/µL) in comparison to group II (846 ± 2.73 × 103/µL) (P value IV:II < 0.0001 and P value VI:II < 0.0001). There were no significant differences after 10 days in groups I (851 ± 3.06 × 103/µL), III (848 ± 4.50 × 103/µL), and V (846 ± 5.60 × 103/µL) (P value III:I = 0.24 and P value V:I = 0.05).
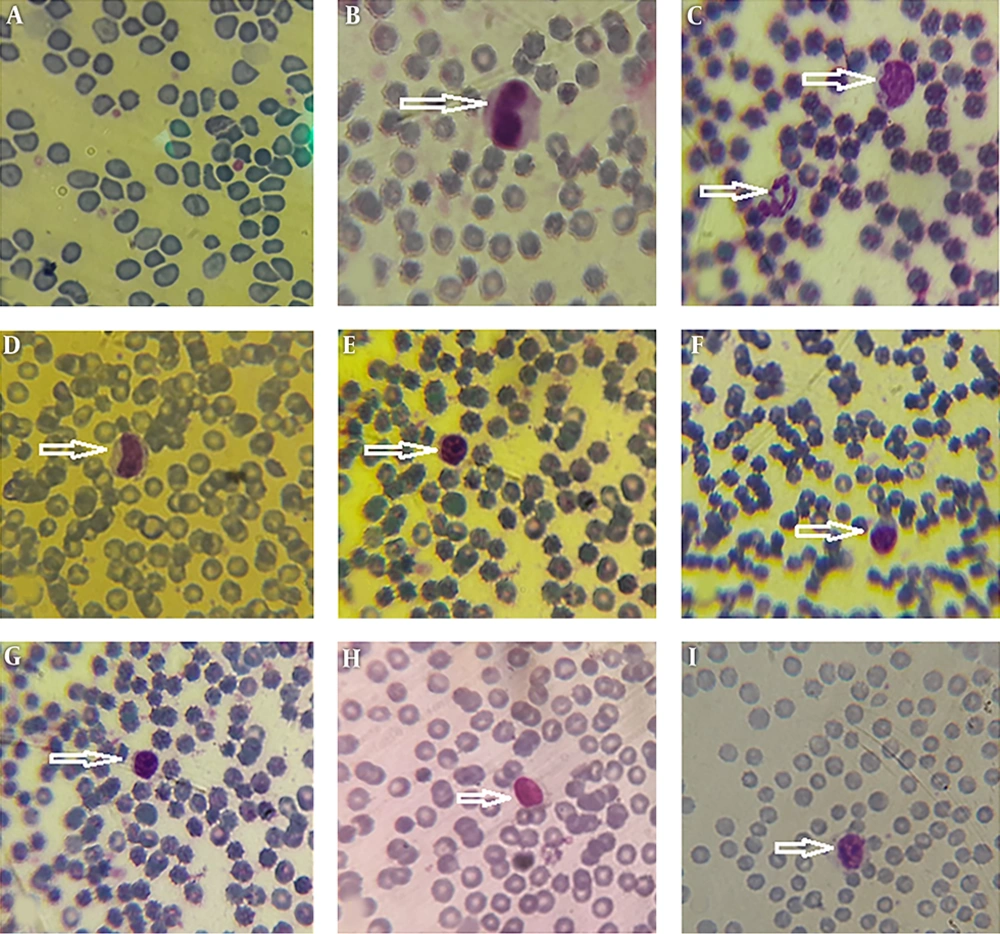
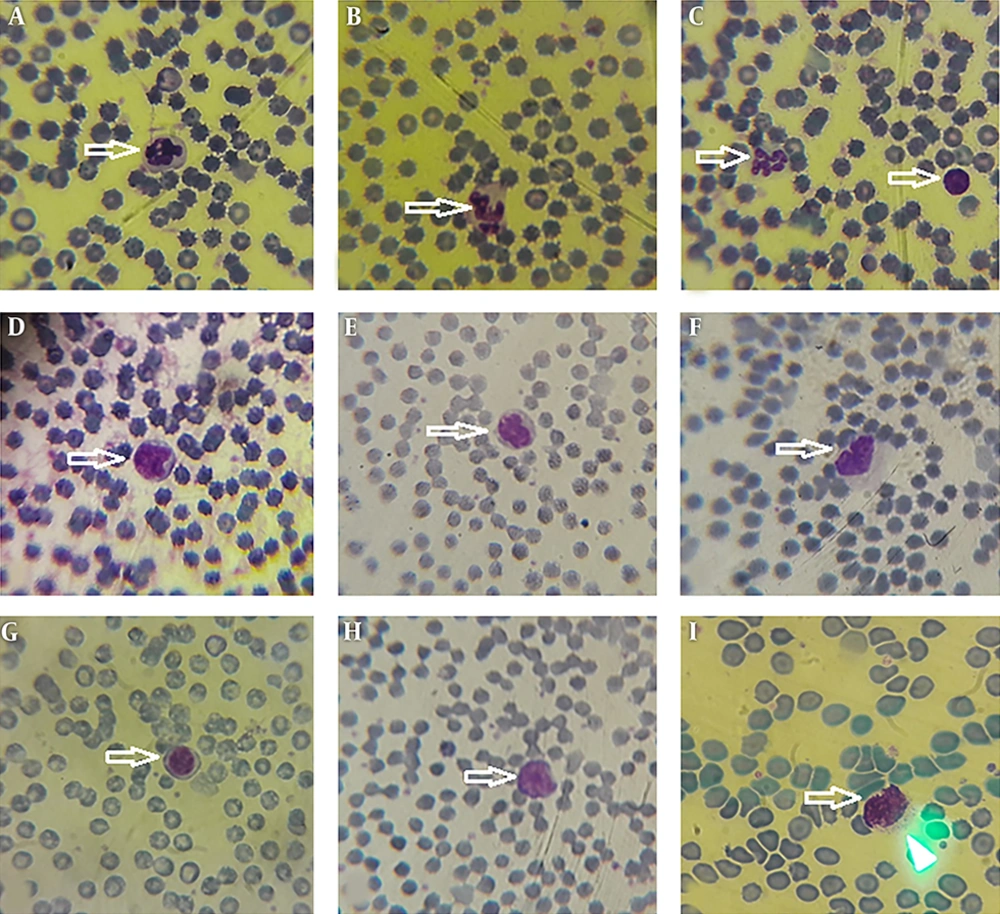
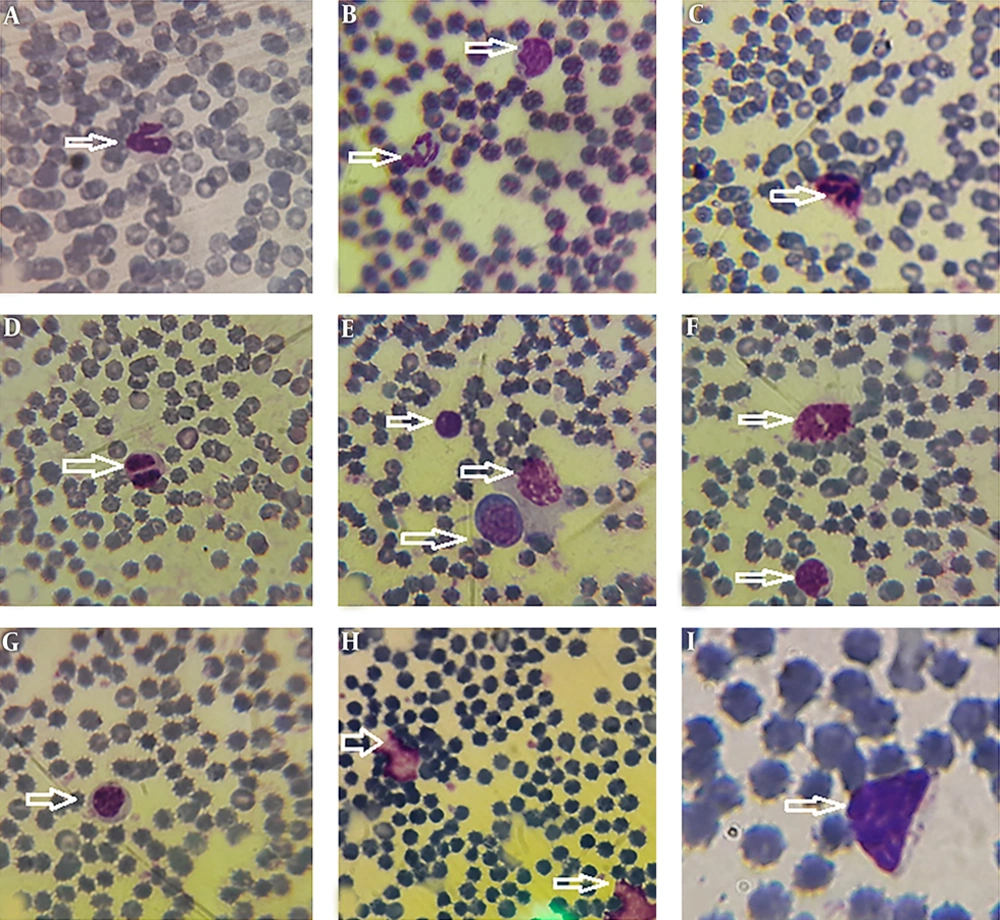
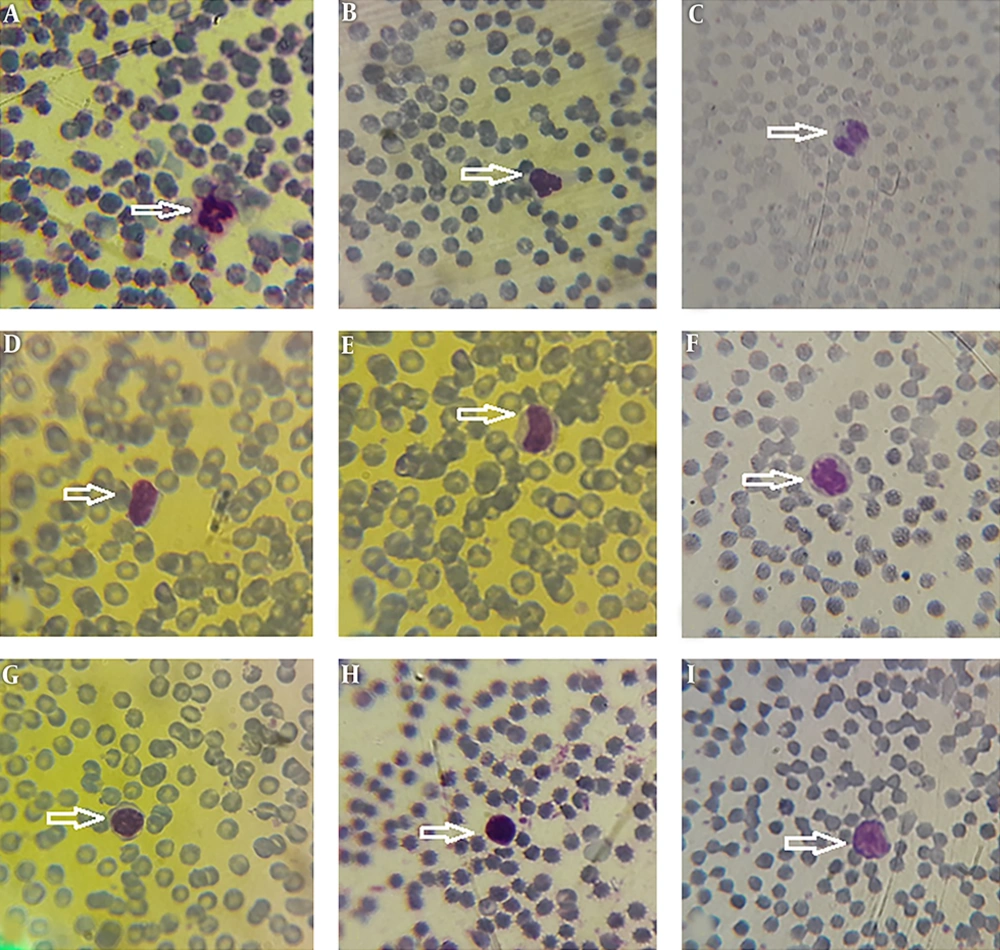
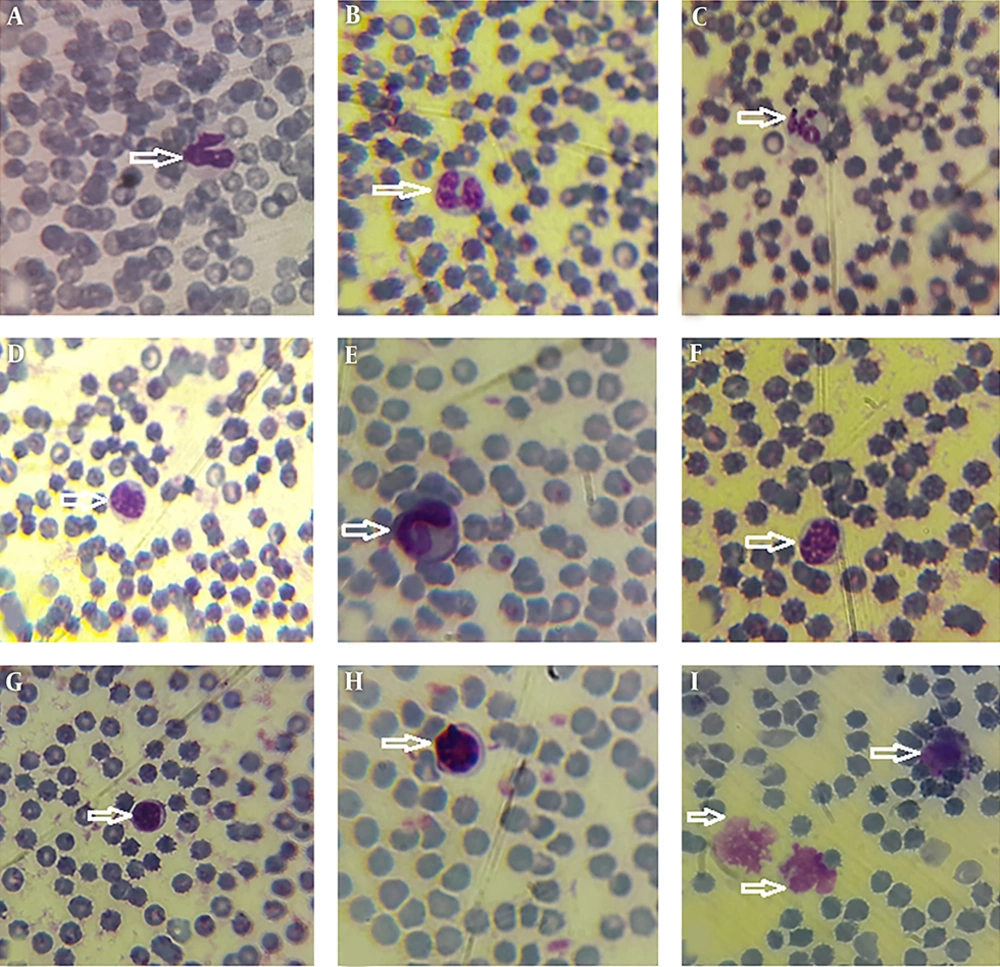
Morphological data of this study in the control group without CFLs exposure showed that the size and shape of leukocytes and their subtypes were normal. Hemolysis and atopic cells were not observed (Figure 1A-I). The number of neutrophils (Figure 1C), monocytes (Figure 1B-D), and lymphocytes (Figure 1E-I) were observed. After exposure to CFLs, atopic cells, bond cells, and hemolysis of leukocytes were observed after 10 and 30 days (Figures 2 and 3). After exposure to CFLs, atopic cells in lymphocyte was observed after 10 days (Figure 2I). After 30 days, the bond cells (Figure 3A), lysis of monocytes (Figure 3E) and lymphocytes (Figure 3H), abnormal monocytes (Figure 3F) and atopic lymphocytes (Figure 3G and I) were seen, but during treatment with curcumin, there were no changes in leukocytes after 10 and 30 days (Figures 4 and 5). We only observed lysis of lymphocytes after 30 days (Figure 5I).
5. Discussion
The protective effects of curcumin on CFL complications have hardly been studied. The current study showed that exposure to UV radiation causes changes in leukocytes and their subtypes, however, administration of curcumin had a positive effect and led to a normal number and morphology of leukocytes and platelets. It was also found that duration of fluorescent exposure has a positive effect on leukocytes, their subtypes, and the platelets.
In this study, it was revealed that UV radiation generally decreases the number of eosinophils, monocytes, lymphocytes, and platelets. It can be a direct effect of UV radiation emitted by CFLs. Similar findings were suggested by Daga et al. (22). Hemolysis is a type of acute toxicity and rapid process disturbance of blood cells (23). Some studies demonstrated that UV radiation reduces the number and the proliferation of leukocytes in peripheral blood (9). These data also confirm our results. Reduction of leukocytes is probably due to DNA damage, because UV radiation produces free radical production and also initiate DNA damage (5). Our study demonstrated that increased exposure duration has a potent effect on reduction of leukocyte counts. In the current study, the effect of UV radiation on number of neutrophils was different from its effect on the other subtypes and the number of neutrophils in fluorescent groups was higher in comparison with the control group. We recommend further studies in order to detect the exact effect of UV radiation on neutrophil count.
In this study, after administration of curcumin, number and morphology of leukocytes were changed into normal status. Curcumin is a plant-derived natural antioxidant with an extensive application in traditional medicine and numerous pharmacological activities (24). Curcumin could be used as a non-genotoxic agent reducing the DNA damage, impeding reactive oxygen species generation, having protective effect against free radicals, and raising the level of antioxidant activity (25). A study has demonstrated that curcumin may elevate the expression of antioxidant enzyme, inhibit reactive oxygen species, and protect effect of oxidative stress (26). Several studies have demonstrated that curcumin induces apoptotic cell death in various types of cancers (27, 28). This apoptotic cell death was mitochondria-dependent path way (29). In our study we also observed some exceptions. The numbers of eosinophils and monocytes after administration of curcumin in addition to 30-day exposure to UV radiation were lower than their counts in groups only exposed to UV radiation. Further studies with higher populations would be helpful to understand the etiology of this finding.
The morphological pattern of this study showed that fluorescent light cause abnormality, hemolysis, and bond cells in leukocytes. A study conducted by Kaestner et al. also showed that UV radiation leads to hemolysis of RBCs (30). Some studies demonstrated that UVC radiation changes erythrocyte membrane (31). Disturbance of the membrane causes penetration of lytic component and collection of water into the cell lead to hemolysis (31). A similar result was seen in our study.
Diseases involving hemolysis are often associated with coagulability (32). Therefore, activation of coagulation process causes reduction of platelets in peripheral blood circulation. Our data also revealed that CFL exposure decreases the number of platelets.
In conclusion, we demonstrated that exposure to UV radiation reduces leukocyte and platelet counts and causes the production of bond and atopic cells, and lysis of leukocytes. Curcumin may have preservative effect on the mentioned complications evoked by CFLs. Due to the small sample size of our study, we recommend further studies in this field, especially the studies that also investigate the hematological effects of curcumin on control rats.