1. Background
Obesity is known as the main cause of many problems like hyperlipidemia, diabetes type 2, cancers, cardiovascular and psychological disorders, high blood pressure (hypertension), sleep apnea, musculoskeletal disorders and significant decrease in quality of life (1-7). Laparoscopic sleeve gastrectomy surgeries are proposed as one of the procedure to achieve efficient weight loss for morbidly obese patients nowadays (8). Sleeve gastrectomy is one of the well-known restrictive procedures for morbid obesity. In this surgery, the stomach is dissected along the greater gastric curvature, and gastric volume reduced, and finally, a tubular gastric pouch is constructed (9). Complications and morbidity with sleeve gastrectomy guided researchers to find new restrictive bariatric surgical procedures. Recently, sub-sleeve gastrectomy has introduced as the experimental technique in which the surgeons resected only the fundus of the stomach (10). Leptin and ghrelin are two important hormones for regulation of weight (a gastric hormone that stimulates appetite). The level of these hormones could be related to gastrectomy. In one study, a decrease was shown in the leptin and ghrelin levels after sleeve gastrectomy (11). By restriction of the stomach capacity, the decrease of ghrelin level was seen (12). Fundus resection could be effective, but it has not been evaluated completely, and there are no noticeable studies on complications, comorbidities, hormonal changes and weight loss after this surgery. If fundus resection changed the hormones and decreased the weight similar to sleeve gastrectomy, lower length of resection in the stomach might cause fewer side effects of the stapler line.
2. Objectives
This study aimed to investigate the effect of sleeve gastrectomy and fundus resection in a suitable animal model (rabbits) to monitor the body weight, and endocrine profiles by measuring fasting ghrelin, and leptin plasma levels. Also to investigate whether Fundus resection (the part of the stomach that releases ghrelin and leptin) is as effective as sleeve gastrectomy on weight loss and hormonal levels or not.
3. Methods
Thirty male New Zealand White rabbits were purchased from a local breeder (Animal Laboratory, Shiraz University of Medical Sciences, Shiraz, Iran). The animals were kept in individual cages by standard temperature, humidity, light, food, and tap water.
Rabbits were randomized into weight-matched three groups. Finally, 21 rabbits divided into three groups, sleeve gastrectomy, fundus resection, and controls (sham-operated) (n = 7). Nine rabbits died during and after surgery because of Ketamine usage as an anesthesia or infection even after Piperacillin consumption and excluded from our study. All animals were given the same amount of food during the experiment to measure weight-loss after the surgeries.
The Ethics Committee approved the study for Animal Research at Shiraz University of Medical Sciences (no. IR.SUMS.REC.1395.S1083).
3.1. Surgical Procedures
After 48 hours of fasting with an empty stomach, the rabbits were anesthetized by ketamine (50 mg/kg) and xylazine (10 mg/kg) intramuscularly. Piperacillin (100 mg/kg) was administered immediately after the surgery, and three following days.
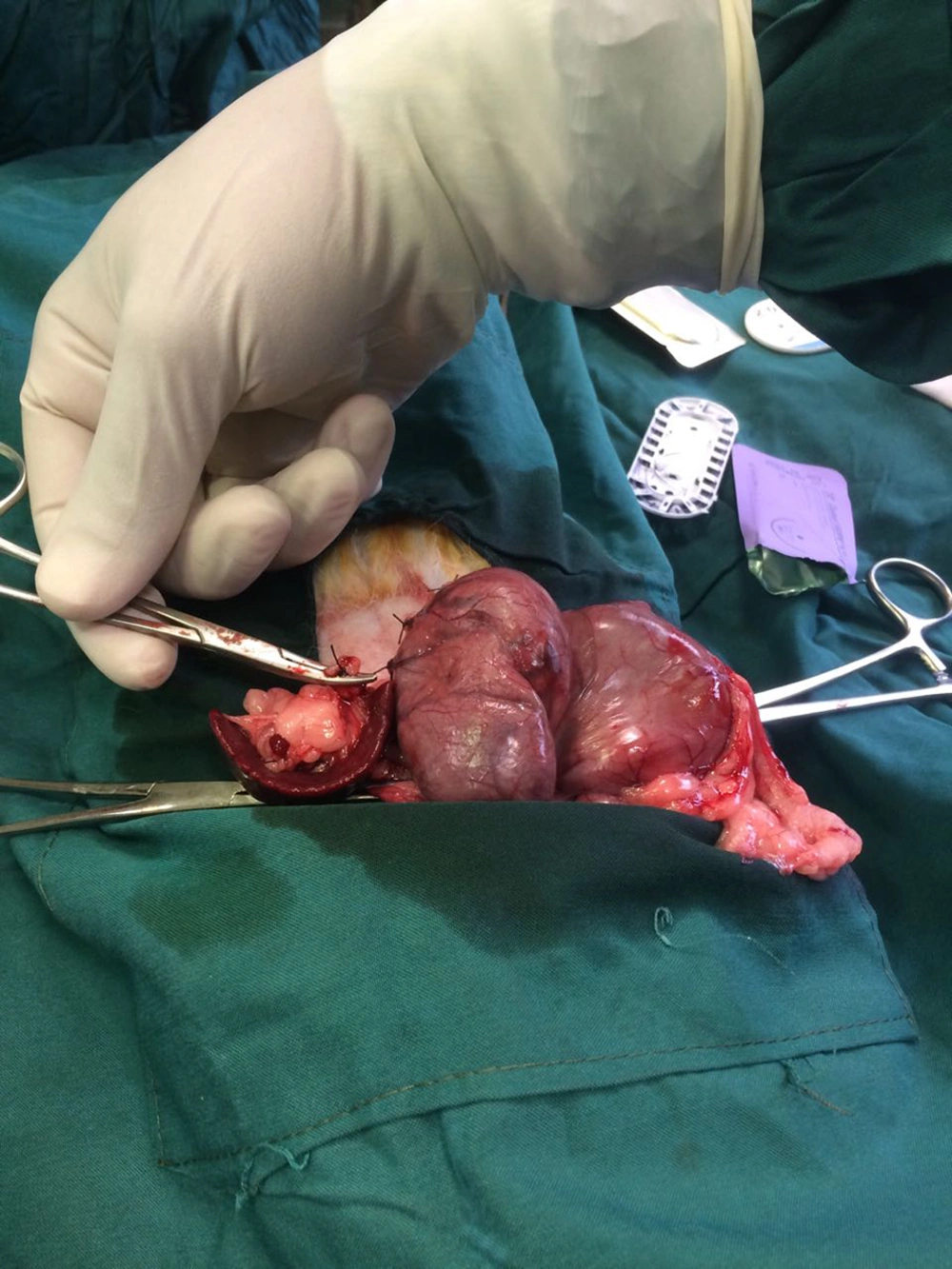
In the sleeve gastrectomy group, approximately 2 cm upper midline was incised as well as the stomach was pulled out; then, a clamp was placed along the greater curvature across the stomach for attaining the same size and approximately 80% of the glandular stomach was removed by scissor (Figure 1). The gastric tube as sleeve gastrectomy fixed by closed with 3 - 0 absorbable polyglyconate suture (Vicryl, Ethicon).
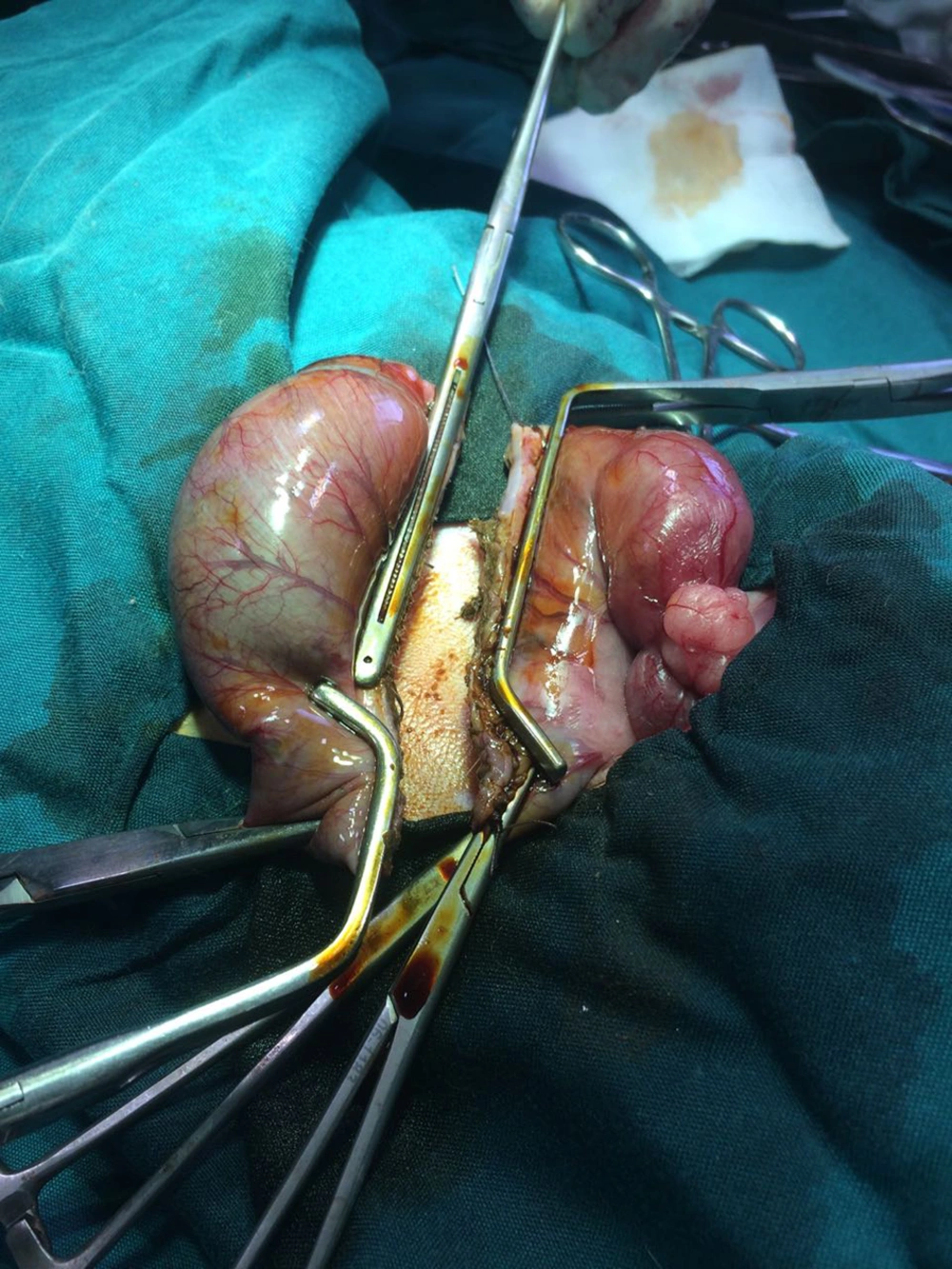
The fundus resection performed the incision, we externalized the stomach. However, the fundus of the gastric curvature was excised and closed by a 3 - 0 absorbable polyglyconate suture (Vicryl, Ethicon). In the resection of the fundus, about 40% of gastric volume was excised, and in the sham-operated group, after a midline incision, the stomach was pulled out, manipulated for 5 minutes, and then returned to the abdomen (Figure 2).
All the rabbits were allowed to return to their cages and recovered from surgery. Their diet was restricted to water and sugar (20 mg) for the first postoperative day, and for the three following recovery days, the diet was replaced by milk and multi-vitamin (5 drop/500 cc milk). They had access to standard rabbit chow until the end of the study; it means 12 weeks.
Bodyweight was measured 3 times; before surgery, one month and 3 months after surgery. Hormonal study for the level of ghrelin and leptin was examined just before the surgery, three months after the procedure in 48 hours fasting condition. Their blood centrifuged at 2,800 rpm for 10 min at 4°C and supernatant was separated and kept at -70°C until the assays were performed. ELISA determined plasma levels of total ghrelin (E0012Rb, Bioassay technology laboratory, Shanghai, China) and leptin (E0173Rb, Bioassay technology laboratory, Shanghai, China).
3.2. Statistical Analysis
Data are shown as means ± SEM of weight, leptin and ghrelin plasma levels before and after the surgery. Kruskal-Walis test was performed for comparison of the means between the groups, and the difference after months in one group and repeated measurement test during the time was used. P < 0.05 was considered to be statistically significant.
4. Results
Rabbits undergoing sleeve gastrectomy displayed a significant weight loss into one month after surgery when compared to fundus resection and sham-operated controls (P = 0.008), while, the fundus resection group has weight gain after 12 weeks (P = 0.020; Table 1).
| Groups | Weight Before Surgery (g) | Weight After One Month (g) | Weight After Two Months (g) | Weight After Three Months (g) | P Valueb |
|---|---|---|---|---|---|
| Fundectomy | 2870.0 (2742.5 - 3028.7) | 2940.0 (2865.0 - 3202.5) | 3050.0 (2937.5 - 3262.5) | 3200.0 (3067.5 - 3395.0) | 0.020 |
| Sleeve | 2640.0 (2277.5 - 3055.0) | 2220.0 (2125.0 - 2315.0) | 2675.0 (2472.5 - 2997.5) | 2720.0 (2460.0 - 3197.5) | 0.058 |
| Sham | 2987.5 (2882.5 - 2998.7) | 3127.5 (2972.5 - 3338.7) | 3350.0 (3060.0 - 3400.0) | 3500.0 (3267.5 - 3575.0) | 0.082 |
| P valuec | 0.468 | 0.008 | 0.05 | 0.034 |
aValues are expressed as median (IQR).
bFriedman test.
cKruskal-Wallis test.
There was a significant decrease in leptin level before and after fundus resection after 3 months (P = 0.025; Table 2). There were no statistical differences in the fasting total ghrelin plasma levels between sleeve gastrectomy, fundus resection and controls after three months (Table 3). A decrease was seen in the ghrelin plasma levels of rabbits undergoing fundectomy (P = 0.076), but it was not statistically significant and it might be related to the small sample size.
| Groups | Leptin Before (ng/mL) | Leptin After One Month (ng/mL) | Leptin After Three Months (ng/mL) | P Valueb |
|---|---|---|---|---|
| Fundectomy | 39.96 (15.49 - 67.05) | 21.38 (12.75 - 63.95) | 24.88 (15.99 - 77.82) | 0.025 |
| Sleeve | 45.64 (17.75 - 80.05) | 42.70 (16.46 - 75.35) | 58.70 (30.30 - 81.20) | 0.779 |
| Sham | 46.31 (18.12 - 87.52) | 54.30 (20.99 - 63.00) | 35.40 (13.79 - 58.17) | 0.105 |
| P valuec | 0.512 | 0.590 | 0.190 |
aValues are expressed as median (IQR).
bFriedman test.
cKruskal-Wallis test.
| Groups | Ghrelin Before (pg/mL) | Ghrelin After One Months (pg/mL) | Ghrelin After Three Months (pg/mL) | P Valueb |
|---|---|---|---|---|
| Fundectomy | 70.72 (38.82 - 98.82) | 62.61 (49.10 - 93.82) | 60.29 (35.07 - 84.42) | 0.076 |
| Sleeve | 60.45 (43.15 - 86.02) | 75.76 (52.08 - 93.43) | 65.40 (41.90 - 97.60) | 0.223 |
| Sham | 82.19 (58.14 - 103.82) | 77.60 (61.01 - 98.55) | 62.85 (40.37 - 86.27) | 0.174 |
| P valuec | 0.491 | 0.593 | 0.560 |
aValues are expressed as median (IQR).
bFriedman test.
cKruskal-Wallis test.
5. Discussion
Sleeve gastrectomy is a restrictive surgery that is effective as a single procedure in patients with body mass index (BMI) > 40 or co-morbid patients with BMI > 35 (13, 14). Fundus resection or sub-sleeve gastrectomy is a new experimental technique that its effectiveness in improving weight and comorbidities has not yet been extensively characterized. Our study indicate the rabbits to represent sleeve gastrectomy and fundus resection procedures in order to investigate the endocrine alternations and weight loss induced by surgery. In our results, similar to previous reports, rabbits undergoing sleeve gastrectomy showed a significant decrease in the body weight after one month when compared with sham-operated control animals and fundectomized rabbits, suggesting that weight loss was more effective in this group (Table 1). This reduction could be related to the extension area of resection more than the reduction of ghrelin and leptin in sleeve gastrectomy. There was a significant decrease in the leptin levels in the fundectomy group (Table 2).
Based on previous studies, sleeve gastrectomy decreased the leptin and ghrelin levels (13-15). Plasma ghrelin levels usually had increased in body mass index (16). On the other hand, sleeve and sub-sleeve surgeries resected the gastric fundus, which secret the main amount of ghrelin (12). In one study in gastrectomized mice replacement of ghrelin reversed the weight loss and increased body fat (17). In another study about obese dogs, the plasma level of leptin and ghrelin was measured after a special diet due to weight loss. The ghrelin level lowered, and the leptin level became higher in comparison to normal-weight dogs, and the low-calorie diet made a decrease in the leptin level, but there was no change in the ghrelin level (18). However, in some studies like ours, there were variable changes in plasma ghrelin levels following sleeve gastrectomy and fundus resection (19). In another study, only in the first six postoperative months ghrelin levels were decreased after sleeve gastrectomy and after that did not have change significantly (12). In obese, leptin-resistant Zucker rats, ghrelin levels were unchanged 14 days after sleeve gastrectomy and weight loss after surgery did not show any correlation with ghrelin level (20). Also, in our investigation, total plasma ghrelin levels were not significantly different from the controls after the surgery as well. It suggested that weight loss after one month induced by sleeve gastrectomy is not dependent on ghrelin secretion. Other hypotheses indicate a compensatory increase in ghrelin secretion with other parts of the stomach in rabbits or by extra gastric ghrelin sources (21). Sectioning of the vagus nerve in surgical technique, may also contribute to changes in ghrelin secretion, because the rabbits which underwent sleeve gastrectomy did not show any difference in the leptin levels, opposite to previous studies (13, 22). In one study, rats that underwent sleeve gastrectomy significantly reduced their food intake in response to leptin. Basically, the leptin’s effects on controlling food intake were mediated by the hypothalamic melanocortin pathway but they did not find any evidence for increased activity of the leptin-melanocortin axis after sleeve gastrectomy (22). In our study, the weight of rabbits after fundus resection did not change significantly and fundus resection seems to be inefficient as sleeve gastrectomy but it reduced leptin level significantly after 3 months (P = 0.025). In this regards, fundectomy could be suggested as a new option in metabolic disorders due to the high level of leptin, and the ability of sleeve gastrectomy to result in weight loss is based on some other effect rather than endocrine hormones. Finding out these effects will provide important insights into a new aspect of both surgical and non-surgical, could be designed.
5.1. Conclusions
Reduction of gastric volume and weight loss can be obtained by sleeve gastrectomy but do not affect the leptin and ghrelin plasma levels significantly. In conclusion, fundus resection is a new bariatric surgery, but it was not efficient as much sleeve gastrectomy as regard to weight loss. Moreover, sub-sleeve gastrectomy suggested as a new option in metabolic disorders due to the high plasma level of leptin.