1. Background
Acetaminophen and/or N-acetyl-p-aminophenol (APAP) is commonly administered as an analgesic drug worldwide. At remedial doses, APAP is predominantly eliminated by phase 2 enzymes to inert conjugates. A small amount of this medicine being metabolized by cytochrome P450 (CYP2E1) to the extremely reactive agents, N-acetyl-p-benzoquinoneimine (NAPQI). NAPQI is eliminated by combining with glutathione (GSH) to create an inert conjugate. The saturated phase-2 pathways can lead to high NAPQI at APAP overdose, GSH depletion, and hepatic dysfunction (1, 2).
In recent years, developing countries provide their health needs with herbal medicine (3). Belonging to the Amaryllidaceae family, the Allium genus has about 700 different species, such as Allium cepa (onion), A. schoenoprasum (chive), A. sativum (garlic), A. tuberosum (garlic chive). Their antioxidant potential is due to the presence of thiosulfinates and other organosulfur, saponins, flavonoids, and phenolic compounds, which are nutritionally and economically valuable (4).
According to recent meta-analyses studies, these species have beneficial effects on blood pressure, lipid profiles, and other cardiovascular risk factors (5-8).
2. Objectives
The A. hooshidaryae is a native plant in the Kurdistan Region, consumed by local people as an herbal medicine for various diseases. Given the limited evidence regarding the therapeutic effects of A. hooshidaryae, the present study aimed to investigate the possible protective effects of methanolic extract of this plant on the hepatotoxicity induced by APAP.
3. Methods
3.1. Chemicals
N-acetyl-p-aminophenol (APAP, CAS: 103-90-2), 2-thiobarbituric acid (TBA), 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB), N-(1-naphthyl) ethylenediamine dihydrochloride (NED), sulfanilamide, and 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) were bought from Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
3.2. Plant Material and Extraction
The A. hooshidaryae was collected from Iran, Prov. Kordestan, (Hawraman Mountains), and identified by herbarium unit, Natural Products Research Center (NPRC), Hamadan, Iran (NO: 274). The aerial parts of A. hooshidaryae were dried in the shade and soaked in equal proportions of water and methanol after crushing for three days. The extract was concentrated by a rotary evaporator at 45°C and then fractioned by column chromatography by n-hexane, chloroform, ethyl acetate, and methanol fractions. For this purpose, a silica gel column (30 - 70 mesh) was used as a solid phase. The quality of fractions was evaluated by the ferric reducing/antioxidant power (FRAP) method.
3.3. Animals
The animal study protocol was approved by the HUMS Ethics Committee (identification code: IR.UMSHA.REC.1396.365). Male Wistar rats (250 ± 20 gram) were purchased from the animal center of HUMS and kept for one week under suitable conditions (temperatures 22°C - 25°C, 12 h light/dark cycle) and fed with a commercial laboratory diet and water ad libitum.
3.4. Preliminary Studies
The preliminary studies revealed that the A. hooshidaryae methanolic extract (AhME) has the highest antioxidant potential and was safe in the dose range of 50 - 200 mg/kg.
3.5. Study Design
Thirty-six rats were divided into six groups of six each. Groups 1 (negative control) and 2 (positive control) received normal saline; groups 3 received 200 mg/kg of AhME, and groups 4 - 6 received AhME at the doses of 50, 100, and 200 mg/kg once daily, by gavage, respectively. After the 14 days, except for groups 1 and 3, the other groups received 2 g/kg of APAP. After 48 hours, the rats were anesthetized using ether and killed. The blood sample was collected from the rat’s heart and centrifuged (3000 rpm, 10 min). The serum sample was removed and kept at -20°C. In addition to liver tissue sampling for histological studies, a small portion of hepatic tissue (100 mg) was homogenized in 1 mL phosphate-buffered saline (PBS, pH = 7.4). The liver homogenate was centrifuged (4000 rpm/4°C/5 min), and its supernatant was separated and kept at -80°C until the biochemical evaluation.
3.6. Serum Enzymes Analysis
The serum alanine transaminase (ALT), lactate dehydrogenase (LDH), aspartate transaminase (AST), and alkaline phosphatase (ALP) values were measured kinetically using commercial kits (Pars Azmoon, Iran).
3.7. Assessment of Hepatic Oxidative Injury
In the hepatic tissue, nitric oxide (NO) was detected using Griess reagent as described by Nili-Ahmadabadi et al. (9). Also, total antioxidant capacity (TAC) and lipid peroxidation (LPO) were measured by FRAP and thiobarbituric acid reactive substances (TBARS) methods, as described in the previous study (9, 10). Total thiol groups (TTGs) were determined via DTNB as the reagent (11). The GSH hepatic level was assayed based on the kit protocol from ZellBio GmbH Company, Germany. Finally, protein content was assayed in tissue homogenate supernatant via the Bradford method.
3.8. Histopathological Consideration
A piece of liver tissue was held in 10% neutral buffered formalin for at least 24 h to be fixed. The paraffin-embedded block was prepared and cut into four µm thick sections by a microtome instrument. After staining with hematoxylin and eosin (H & E) dye, stained samples were evaluated under the light microscope (Olympus CX31 microscope) (12).
3.9. Statistical Analysis
The data is described as the mean ± SEM. Differences were determined at 95% confidence (P-value < 0.05) via analysis of variance (ANOVA) and Tukey’s posthoc test. The statistical analysis was conducted using SPSS version 20 (IBM, Armonk, NY, USA).
4. Results
4.1. The Effects of AhME on Serum Liver Enzymes
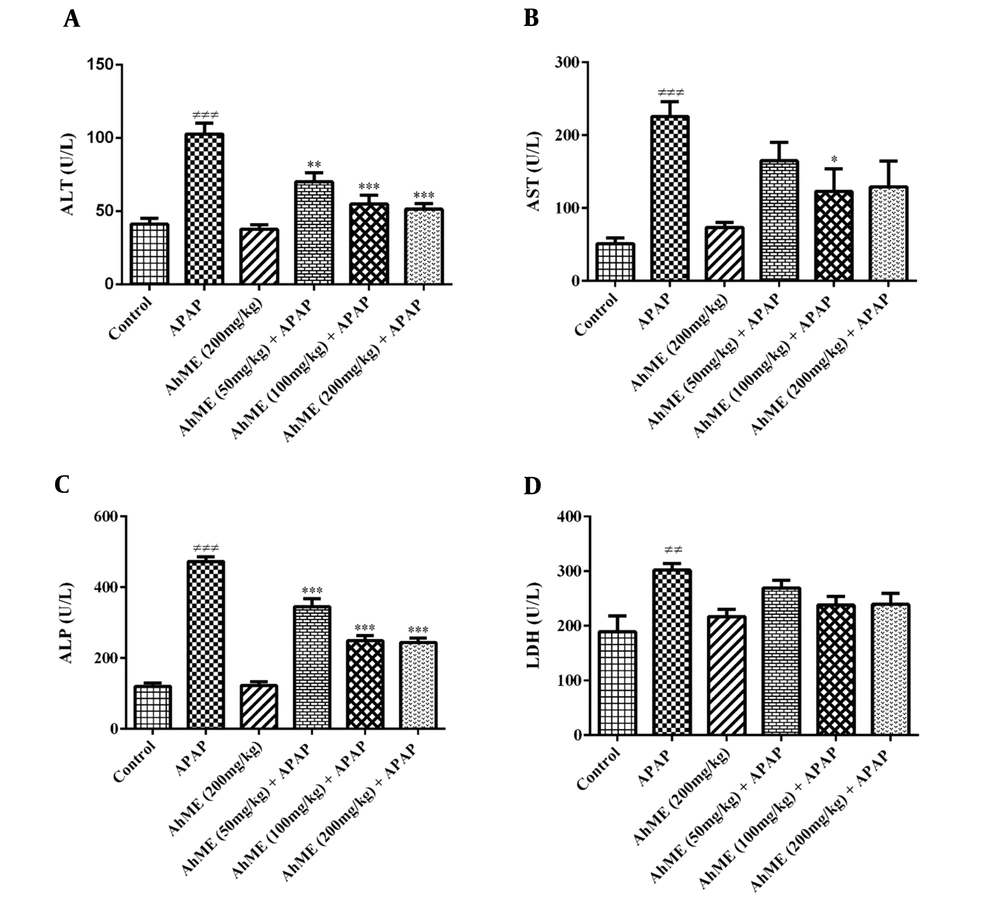
The findings showed no sign of toxicity up to 200 mg/kg of AhME. As shown in Figure 1, the APAP notable increased AST (P < 0.001), ALT (P < 0.001), LDH (P < 0.01), and ALP (P < 0.001) serum activity when compared to control group. The AhME decreased the serum levels of hepatic enzymes (AST, ALT, and ALP) in rats exposed to APAP. There was no significant change in serum LDH activity in the pretreatment groups compared to the APAP group.
Effects of Allium hooshidaryae methanolic extract (AhME) on serum enzymes of acetaminophen (APAP)-exposed Wistar rat. Statistical analysis used one-way ANOVA with Tukey’s test. Values are expressed as means ± SEM, n = 6 for each group. ###, P < 0.001 vs control group; *, P < 0.05, **, P < 0.01 and ***, P < 0.001 vs APAP group. ALT, alanine aminotransferase (A); AST, aspartate aminotransferase (B); ALP, alkaline phosphatase (C); LDH, lactate dehydrogenase (D).
4.2. The Effects of AhME on Oxidative Stress Markers
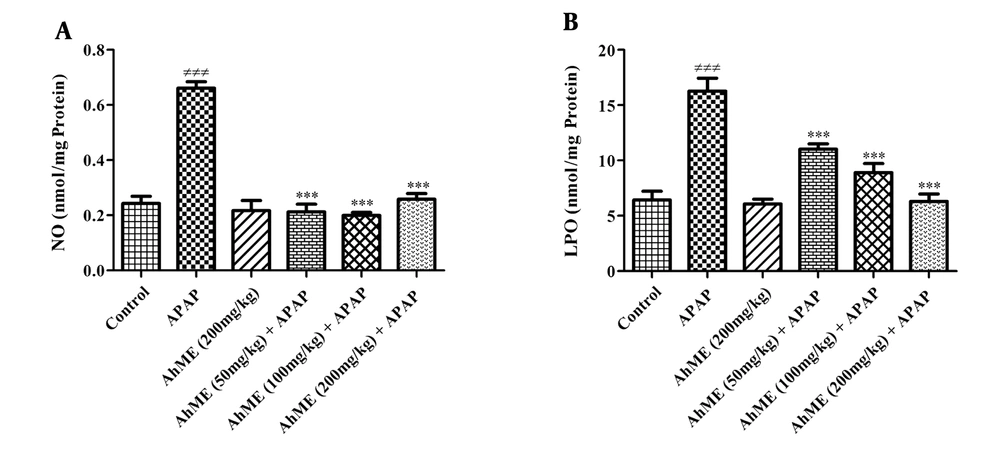
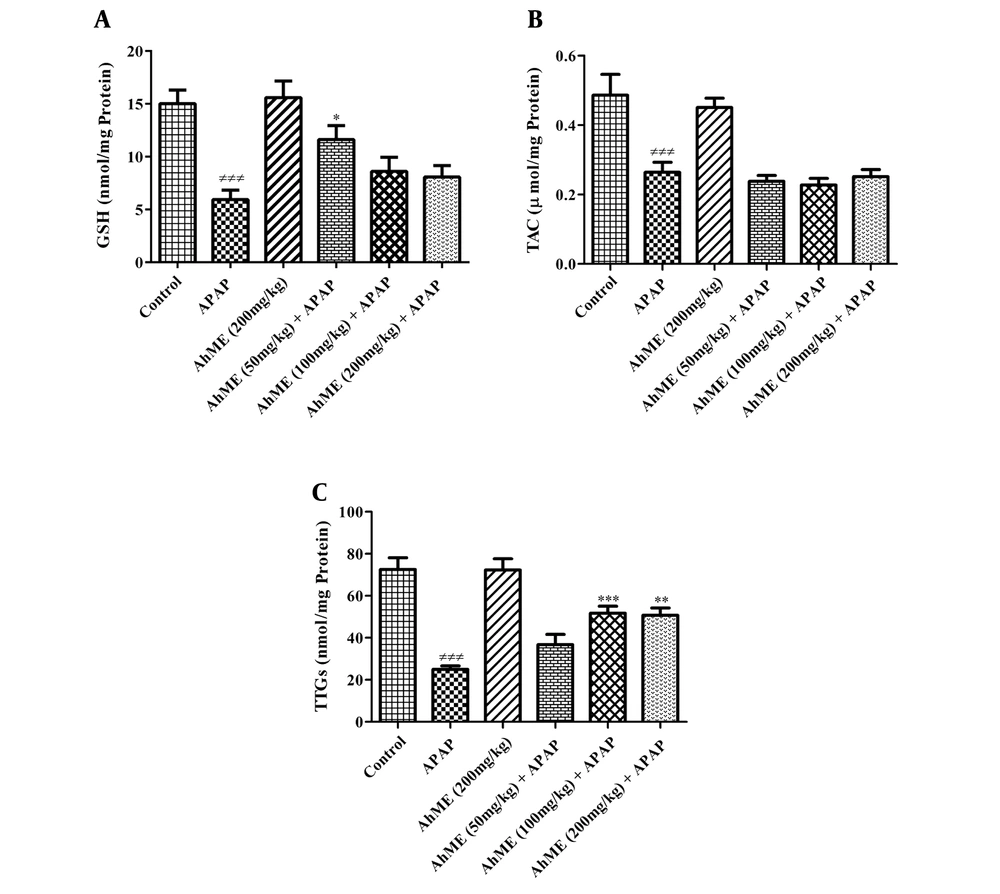
After APAP administration, the levels of LPO and NO were increased (Figure 2, P < 0.001). Also, GSH, TAC, and TTGs levels were decreased in hepatic tissue compared to the control group (Figure 3; P < 0.001). Following pretreatment with the AhME, a significant improvement was observed in hepatic TTGs, especially at doses of 100 and 200 mg/kg compared to the APAP group (P < 0.001 and P < 0.01, respectively). Besides, administration of AhME can improve the GSH level at the dose of 50 mg/kg (P < 0.05) and NO and LPO levels (for all doses, P < 0.001) in hepatic tissue compared to the APAP group.
Effects of Allium hooshidaryae methanolic extract (AhME) on oxidant biomarkers of acetaminophen (APAP)-exposed Wistar rat. Statistical analysis used one-way ANOVA with Tukey’s test. Values are expressed as means ± SEM, n = 6 for each group. ###, P < 0.001 vs control group; ***, P < 0.001 vs APAP group; NO, nitric oxide (A); LPO, lipid peroxidation (B).
Effects of Allium hooshidaryae methanolic extract (AhME) on antioxidative biomarkers of acetaminophen (APAP)-exposed Wistar rat. Statistical analysis used one-way ANOVA with Tukey’s test. Values are expressed as means ± SEM, n = 6 for each group. ###, P < 0.001 vs control group; *, P < 0.05, **, P < 0.01 and ***, P < 0.001 vs APAP group. GSH, glutathione (A); TAC, total antioxidant capacity (B); TTG, total thiol group (C).
4.3. Histopathological Alterations
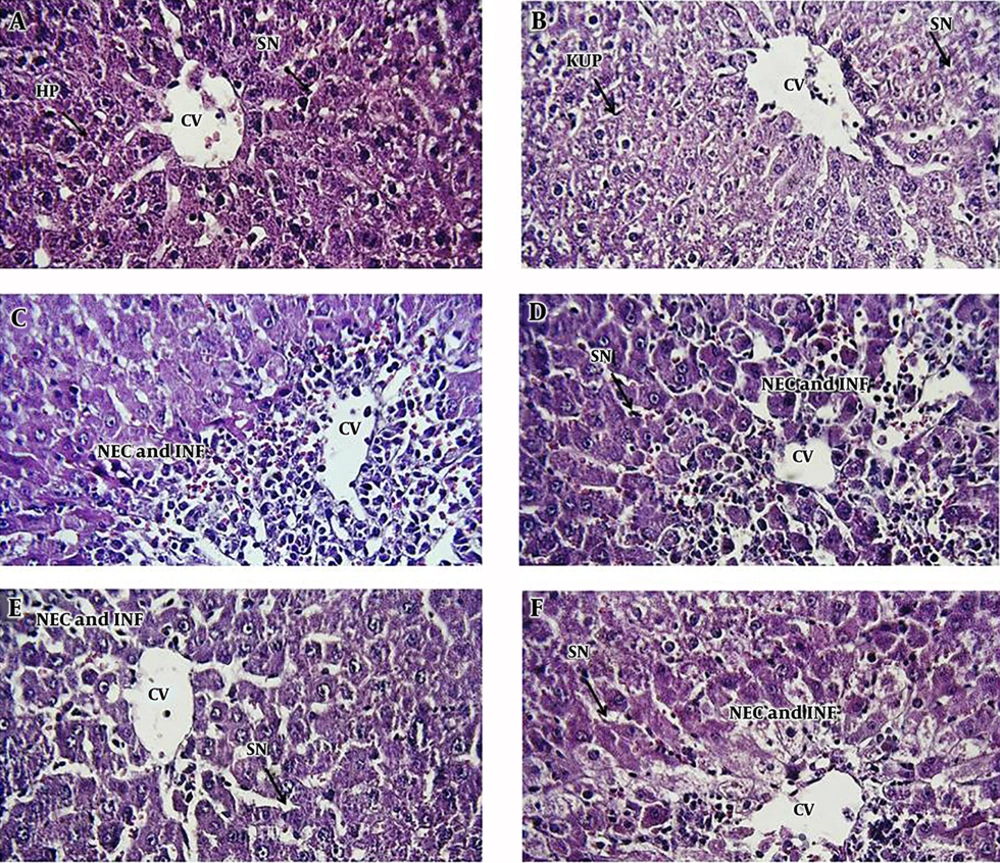
We observed the vascular congestion, mononuclear cell infiltration, necrosis, dilation of sinusoids, and increasing kupffer cells in the liver of APAP-treated rats. Following pretreatment with different doses of AhME, a noteworthy improvement was found in some of the pathological alterations like necrosis and vascular congestion (Figure 4).
Photomicrographs of rat liver tissue in different groups: A, negative control group; B, AhME control group; C, acetaminophen (APAP; 2 g/kg); D, AhME (50 mg/kg) + APAP (2 g/kg); E, AhME (100 mg/kg) + APAP (2 g/kg); F, AhME (200 mg/kg) + APAP (2 g/kg). Original magnification of all images is 40×. CV, central vein; HP, hepatocyte; SN, sinusoid; KUP, kupffer cells; NEC, necrosis; INF, infiltration.
5. Discussion
Our results showed a notable increase in the serum levels of ALT, AST, and LDH in the APAP-treated rats. These results could be due to increased membrane degradation, leading to enzyme leakage into circulation (13). Also, the increase in serum level of ALP may be due to the biliary tract impairment and cholestasis (14). Pretreatment with AhME could significantly decrease the serum level of hepatic enzymes, which might be due to its membrane stabilizing activity (13, 15, 16).
Since the APAP hepatotoxicity is related to oxidative pathways, hepatic oxidant/antioxidant biomarkers were evaluated. Results showed that TAC, TTGs, and GSH decreased in the hepatic tissue of the APAP group, indicating APAP-induced oxidative damage. These findings were in agreement with the other reports (17-21) related to the altered natural metabolism of the APAP and the disruption of the active thiol groups (22). Overall, APAP is metabolized by the cytochrome P450 enzymes into a reactive metabolite called NAPQI, which reduces the levels of thiol groups and subsequent hepatocellular antioxidant defense (23, 24). GSH is part of the natural antioxidant system that counteracts the oxidative stress caused by free radicals (25). GSH is converted to GSSG (glutathione disulfide) by free radicals, causing apoptosis by excessive oxidation of glutathione (26). Also, APAP-induced hepatotoxicity may be associated with the inhibition of useful enzymes in GSH biosynthesis. Singh et al. (27) found that APAP could inhibit the recovery of oxidized GSH to reduced GSH by inhibiting the GSH reductase enzyme and lead to the depletion of thiol reserves. These changes led to the degradation of macromolecules, such as proteins and nucleic acids, and caused the loss of membrane integrity (28).
Following the administration of AhME, a significant improvement was observed in the level of thiol groups, especially GSH. It seems that Allium species can effectively improve the thiol reserves due to having sulfur compounds. For example, the allicin derivative products (diallyl disulfide, diallyl trisulfide) in garlic essential oils have shown suitable antioxidant activities. Also, the onion has antioxidant potential due to dipropyl disulfide and dipropyl trisulfide (29).
LPO is the degradation of lipid caused by the removal of electrons from the cell membrane by free radicals (30, 31). APAP poisoning can cause the generation of reactive oxygen species (ROS) to leak out of the mitochondria, resulting in LPO. The AhME caused a significant decrease in LPO level due to its membrane-stabilizing properties. In the previous studies, the membrane stabilizing properties of some Allium species have been studied by Sharma et al. (32) and Anitha et al. (33).
NO and its derivatives play crucial roles in liver physiology and pathophysiology. It is mainly made by the endothelial nitric oxide synthase (eNOS) and usually has a protective role against diseases. However, excessive production of NO can result in the overproduction of reactive nitrogen species (RNS) after reaction with superoxide anions and the production of active peroxynitrite species, leading to oxidative damage (34-36). In the present study, APAP increased hepatic NO and significantly reduced this chemical mediator in contrast with AhME. Elevated hepatic NO levels following exposure to APAP may be associated with the induction of nitric oxide (iNOS) activity by this drug (37). It seems that active substances in Allium species such as s-allyl cysteine (SAC) and diallyl sulfide prevent the increase of peroxynitrite level and the occurrence of nitrosative stress by reducing the activity of this enzyme and preventing inflammatory reactions in liver tissue (38, 39).
In conclusion, this data suggests that A. hooshidaryae may prevent APAP hepatotoxicity by improving the hepatic thiol reserves. Therefore, this herbal medicine may be used as a complementary treatment for other liver diseases.