1. Background
More than 70,000 plant species have been used for medicinal purposes. Plant-based herbal medicine, also referred to as botanical medicine or phytomedicine, has been employed in primary healthcare in numerous countries worldwide (1). One of the most prevalent justifications for selecting specific plants for further research is their historical use in the treatment of ailments, which are strongly associated with the clinical and research characteristics of particular diseases (2). Herbal medicine research has increased in various nations owing to a notable increase in public interest and acceptance of natural remedies globally (3, 4).
Despite the extensive history of herbal medicine use, the development of pure compounds from botanical sources presents numerous challenges comparable to those encountered in the development of synthetic molecules employed in contemporary medicine. Consequently, the development of pure substances extracted from herbal materials must be closely monitored and adhere to the good clinical practice (GCP) guidelines as well as the Food and Drug Administration (FDA) criteria for modern medicine. A prime example is the current first-line antimalarial drug artemisinin, derived from the Chinese medicinal plant Artemisia annua. The discovery of artemisinin was awarded the 2015 Nobel Prize in Physiology or Medicine (5, 6).
Ethical conduct is fundamental to both clinical practice and research involving human participants. While clinical ethics primarily focuses on safeguarding the dignity, autonomy, privacy, and welfare of individual patients during diagnosis and treatment, research ethics extends these principles to the broader context of scientific investigation aimed at generating generalizable knowledge (7). Clinical ethics emphasizes respect for patient autonomy, confidentiality, non-maleficence (avoiding harm), beneficence (promoting well-being), and justice in healthcare delivery (8). These principles ensure that patients receive care that honors their rights and protects their safety and privacy.
Research ethics, on the other hand, governs the design, conduct, and reporting of studies involving human subjects. It mandates that research be scientifically valid, socially valuable, and conducted with respect for persons, concern for their welfare, and justice in participant selection (9). Key elements include obtaining informed consent that is fully informed and voluntary, ensuring confidentiality of participant data, minimizing risks, and providing fair access to potential benefits. Ethical oversight by independent review boards is essential to evaluate the risk-benefit balance and protect vulnerable populations (7, 10, 11).
Clinical trials are an essential step in the drug development process, typically progressing from phase 1 to phase 4. A multidisciplinary team with expertise and experience from several fields is required to successfully complete clinical drug development. Adherence to GCP principles is crucial for both the trial subjects' welfare and scientific validity. Ethical considerations are among the most challenging aspects of clinical trials examining crude extracts and fractions from herbal materials (hereafter referred to as herbal drug trials), and many researchers lack confidence in their ability to address such issues (12). The ethical approval process may become more complex because of the difficulties encountered by ethics committees in providing ethical justification for conducting such experiments (13).
To assist clinical investigators and members of ethics committees in navigating the ethical complexities of herbal medication trials, a comprehensive framework that addresses concerns specific to these types of studies is urgently needed. Overall, the regulation of herbal medication is a complex topic with numerous ethical challenges. These issues arise from philosophical disagreements between Western conventional medicine and traditional herbal medicine, the divergent claims of various market participants, the tensions between consequentialist and duty-based ethics, the scarcity of relevant data and guidance, the lack of sufficient supporting evidence, and cultural norms that conflict with ethical principles.
These ethically ambiguous situations may involve moral "grey areas", necessitating weighing the advantages and disadvantages of multiple options, attracting public attention, and involving ethical contradictions. These dilemmas also arise when strong innate intuitions and emotions are involved, potentially resulting in bias. Regulation of the industry has been challenging for regulatory bodies because of these factors (1).
This study aims not only to elucidate the current application of ethical principles in recent research but also to analyze and define the criteria for their significance in clinical trials. This focus is essential for ensuring transparency, justice, and appropriate patient care, elements that are indispensable for advancing medical science and fostering public trust. Examining this topic within a specific five-year timeframe facilitates the identification of key developments and shifts in ethical approaches, which may be rapidly evolving due to a range of social, technological, and institutional factors. Furthermore, this study provides a framework for evaluating the alignment of current regulations with international standards and offers recommendations for enhancing ethical principles and practices in clinical trials to address future challenges.
2. Objectives
Consequently, this research can contribute significantly to increasing awareness and understanding of the most pressing ethical concerns in healthcare and establishing a robust foundation for future policy-making.
3. Methods
This study was conducted retrospectively after receiving approval from the Research Council and the Ethics Committee. We used a census approach to compile a comprehensive list of all clinical trial plans approved in Guilan province from 2019 to 2023, which totaled 199 cases. This was done with the assistance of the university’s research vice-chancellor. For each project, we completed a standardized checklist that included general information such as the year of implementation, gender and field of study of the project manager, drug involved in the study, use of a placebo, age of the study group, and confirmation of informed consent.
3.1. Statistical Analysis
To obtain detailed data, we referred to the clinical registration center website (www.irct.behdasht.gov.ir) as our primary data source. Qualitative variables were expressed as frequency percentages, while quantitative variables were reported as mean values along with standard deviations. Data analysis was carried out using SPSS version 16 software. In this study, the investigation focused solely on the protocols, and the results of these studies were kept confidential. All information extracted from the research proposals was anonymized. Additionally, our reviews were limited to the plans; we did not analyze the final reports of these projects. This study was reported according to STROCSS criteria (14).
4. Results
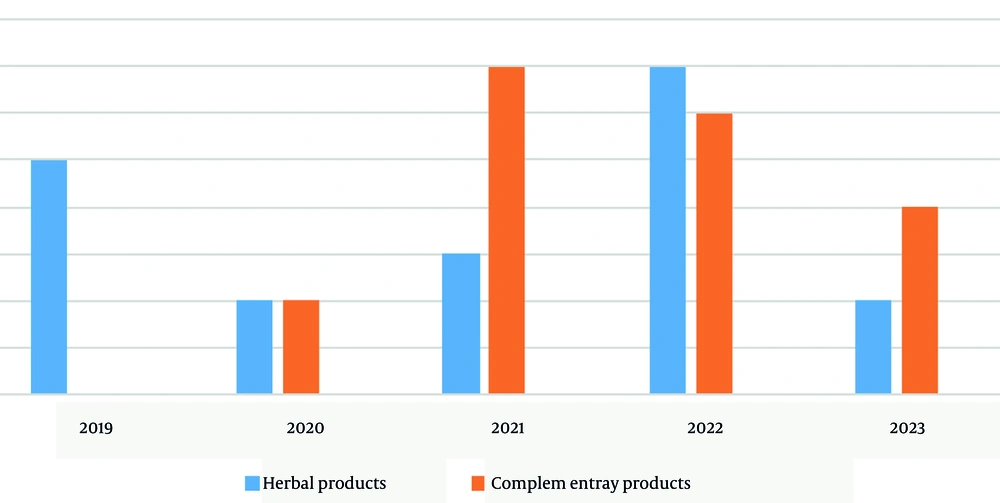
Out of the 199 clinical trials, 19 (9%) focused on herbal products, while another 19 (9%) were related to complementary products. As a result, the number of clinical trials conducted on herbal products is equal to that of complementary products. Also, 68% of the project managers were women and 32% were men. Informed consent was obtained from participants in 29 out of 38 trials (76%). Of the 38 trials examined, 26 (68%) incorporated a placebo. In most trials, one criterion for exclusion was the use of specific medications or the presence of underlying conditions. Only two trials (5%) specified that no other medication was administered during the study.
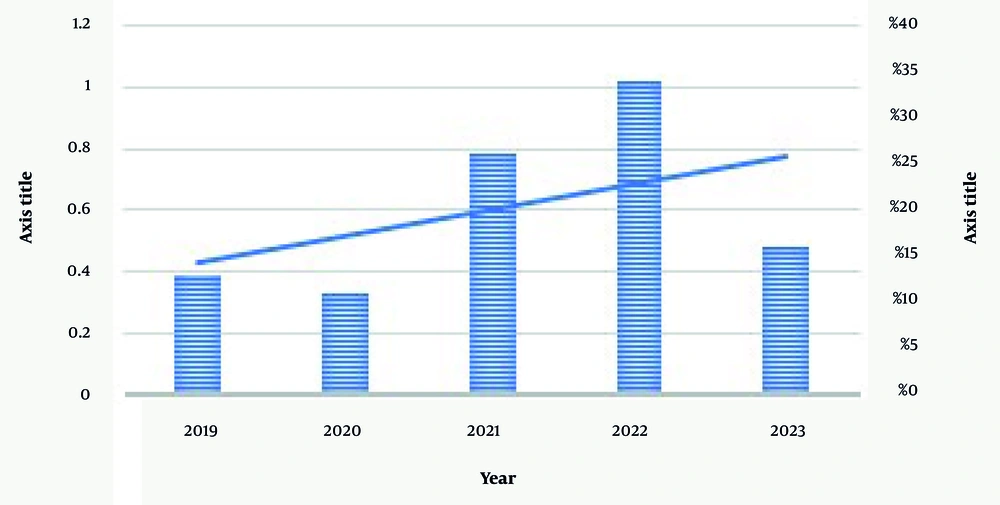
According to Figure 1, there has been an increasing trend in the number of clinical trial research studies. Additionally, Figure 2 illustrated how the number of herbal and complementary products has varied over the years, showing fluctuations and differences in quantity between the two categories for each specific year. Based on the data, complementary products were more prevalent in 2021, while herbal products had a higher presence in 2022 (Figure 2).
Table 1 showed the frequency of fields of study among clinical trial project managers. The pharmacy field had the highest representation, with 8 project managers (21.05%) (Table 1).
| Variables (Project Manager's Field of Study) | Values |
|---|---|
| Medicine | |
| Heart | 2 (5.26) |
| Obstetrics and gynecology | 2 (5.26) |
| Neurology | 2 (5.26) |
| Rheumatology | 2 (5.26) |
| General surgeon | 1 (2.63) |
| Pediatrician | 1 (2.63) |
| Anesthesiologist | 2 (5.26) |
| Otolaryngologist | 1 (2.63) |
| Internal medicine | 2 (5.26) |
| Nutritional medicine | 3 (7.89) |
| Dentistry | 3 (7.89) |
| Pharmacy | 8 (21.05) |
| Nursing | 3 (7.89) |
| Midwifery | 4 (10.53) |
| Other disciplines | 2 (5.26) |
a Values are expressed as No. (%).
Among the herbal products, essential oils and aromatherapy trials accounted for the most research, comprising 26% (Table 2).
| Herb Type | Values |
|---|---|
| Agnugol | 1 (5.2) |
| Lemonade tea | 1 (5.2) |
| Evening primrose oil | 1 (5.2) |
| Turmeric | 2 (10.5) |
| Rose essential oil | 2 (10.5) |
| Aloe vera | 1 (5.2) |
| Black and green tea | 1 (5.2) |
| Fennel capsules | 1 (5.2) |
| Ela ointment | 2 (5.2) |
| Lavender and spring orange essential oil | 1 (5.2) |
| Mint essential oil | 1 (5.2) |
| Rosemary cream | 1 (5.2) |
| Nettle syrup | 2 (10.5) |
| Mouthwash containing green tea and pomegranate peel | 1 (5.2) |
| Lavender essential oil | 1 (5.2) |
a Values are expressed as No. (%).
5. Discussion
Conducting clinical trials in the medical field is of great importance. These trials serve as the final stage in the process of medical research and play a crucial role. On the other hand, adhering to ethical principles in these trials is highly significant. Today, herbal products and supplements have attracted a lot of attention. These types of products are often made from natural ingredients and can even be used as alternatives for treating diseases and enhancing health. Given the potential capacity of herbal medicines and supplements to improve health and treat diseases, more research in the field of herbal products and supplements is necessary.
The results of clinical trials on herbal medicines and supplements in Guilan province, from 2019 to 2023, show that out of 199 trials, only 38 cases (19%) were related to herbal products and supplements. This appears to be a small percentage. An annual review of these 38 trials shows that 13% were in 2019, reaching 34% by 2022. However, in 2023, the frequency of trials is low, which could be due to incomplete registration of the trials conducted before these reviews. Therefore, although the frequency of 19% over 5 years is low, considering the increasing research on herbal medicines and supplements, this figure is acceptable.
In terms of the academic field of the project executors, 47% are in medicine, 21% in pharmacy, and 10% in midwifery. Clinical trials have particular sensitivity and importance from an ethical perspective in certain groups. These specific groups include healthy volunteers, patients with serious illnesses, children, and pregnant or breastfeeding women
Drug clinical trials require these studies to be conducted on healthy volunteers unless the drug under study has serious side effects and risks, in which case the study is conducted directly on volunteer patients. The main ethical issue in studies on healthy individuals requires special attention to voluntary participation and obtaining informed consent. This means that any person intending to participate in the study must do so completely voluntarily and with full awareness. This respects each individual's choice and privacy. Secondly, it is essential to ensure that participants are healthy when entering the study. This is necessary to protect the health and safety of the participants. Therefore, necessary tests and criteria to ensure participants' health should be performed before entering the study, and there should be a maximum effort to avoid exposing individuals to risk. Particularly, elderly people and those predisposed to diseases or health problems should be prevented from participating due to the potential risks associated with the study.
In the conducted review, out of 38 trials, only one was conducted on healthy individuals, and the first condition (voluntary participation) was met. However, this study was specifically conducted on elderly individuals. Clinical trials on severely ill patients also present particular conditions that make decision-making important. In these cases, due to the urgency of the patient's condition, treatment may need to begin as soon as possible. Often in these cases, the individual may not be able to provide consent, and there may not even be a guardian to provide consent. Out of 38 trials, only one trial involved a seriously ill patient, and consent was obtained from a guardian.
Children are a specific group where clinical trials require special attention. The general rule is that studies on children should be limited to problems specific to their diseases. In other cases, such as determining the effective dosage of a drug for children, the intervention's efficacy and safety should have already been proven in studies on adults. Of course, obtaining informed consent from the parents or guardians of the child is necessary, and any lack of cooperation by the child should be regarded as non-consent (16). Out of 38 trials, 6 were specifically conducted on children, and 1 trial was conducted on infants. In the above trials, informed consent was mentioned.
As a general rule, pregnant or breastfeeding women should not be clinical trial subjects unless the trial is designed to protect or promote the health of the pregnant or breastfeeding woman, fetus, or infant. Pregnant women should not be deprived of the benefits that can be derived from evaluations, drugs, vaccines, or other measures that can be useful for treating or preventing their and their fetus's problems (16). Out of 38 trials, only one was specifically conducted on pregnant women.
Based on the results of the review of medical ethics in clinical trials on herbal medicines and supplements in Guilan province from 2019 to 2023, data analysis shows that 76% of the participants in the studies participated with full and informed consent. The results indicate that a significant portion of participants in the trials were fully aware of the goals and methods, and also correctly followed the guidelines (17-19).
However, despite the high percentage of participants who participated with informed consent, the review showed that the results of some trials are still not fully aligned with expectations. Therefore, the causes and problems affecting the complete adherence to medical ethics in these trials should be carefully addressed. To justify and analyze the reasons for non-compliance with complete medical ethics in trials, several factors can be mentioned. Firstly, a lack of awareness among participants about their rights and obligations in trials can lead to incomplete ethical compliance. Also, the absence of precise and comprehensive guidelines related to the complete observance of medical ethics in trials can result in incomplete adherence to these discussions. These analyses indicate that despite the informed consent of individuals in trials and the use of guidelines, more attention is still needed to medical ethics in this field.
5.1. Conclusions
This paper, by examining clinical trials conducted in Guilan province from 2019 to 2023, concluded that most of these trials have proceeded with fidelity to ethical principles. This confirms a strong commitment to ethical standards, which is at the core of contemporary medical research, and beyond that, it reflects respect for the rights of patients and participants in these trials. These results not only demonstrate significant progress towards responsible and attentive conduct in trials but also represent a milestone in ongoing efforts to ensure scientific quality and credibility in the medical field. Thus, the findings of this research promise a favorable environment for advancing the healthcare field with an ethical approach and serve as a worthy model for other research fields where ethics and the advancement of medical knowledge coexist successfully.
5.2. Implications and Recommendations
It is suggested to continue studies on medical ethics in clinical trials on herbal products and supplements in other regions or with different timeframes. Additionally, developing and introducing practical guidelines for observing medical ethics in clinical trials of these types of products can help improve performance and elevate ethical standards in this field.