1. Background
Fluid management in critically ill postoperative patients is crucial to avoid both hypovolemia and fluid overload, which can increase complications and hospital stays. Postoperative patients experience significant cardiovascular alterations due to anesthesia, surgical stress, and inflammatory responses, complicating fluid therapy (1). Optimizing fluid administration remains a challenge in perioperative and intensive care settings. Excessive fluid administration can lead to complications such as pulmonary edema and impaired oxygenation, whereas insufficient fluid resuscitation may result in hypoperfusion and organ dysfunction (2). Therefore, reliable assessment of fluid responsiveness (FR) is critical for guiding appropriate fluid therapy and improving patient outcomes (3).
Traditional static hemodynamic parameters, such as central venous pressure (CVP) and pulmonary artery occlusion pressure (PAOP), have limited predictive value for FR. These parameters fail to account for the dynamic nature of cardiovascular physiology, particularly in mechanically ventilated patients (4). Recent research suggests that dynamic indices, including stroke volume variation (SVV) and ultrasound-based inferior vena cava assessments, offer superior predictive accuracy. However, their clinical applicability remains limited by technical complexity and patient-specific factors (5).
Among dynamic tests, passive leg raising (PLR) is widely used due to its ability to transiently increase venous return, mimicking a fluid bolus without actual volume administration (6). The PLR is simple, non-invasive, and has been validated in various clinical settings. Conversely, the end-expiratory occlusion (EEO) test leverages heart-lung interactions in mechanically ventilated patients to assess preload dependency. The EEO test involves a brief end-expiratory pause, during which the absence of positive-pressure ventilation-induced changes in preload allows for indirect assessment of FR (7).
While both tests have been studied extensively, comparative data regarding their effectiveness in severely ill postoperative patients remain limited. Furthermore, previous studies have primarily focused on critically ill medical patients, leaving a gap in the literature regarding their applicability in surgical populations. Given the distinct hemodynamic alterations encountered in postoperative patients, evaluating the relative predictive value of EEO and PLR is essential (8).
2. Objectives
The present study aims to compare the predictive accuracy of EEO and PLR in assessing FR in mechanically ventilated postoperative patients. By determining the reliability of these tests, our findings may contribute to the optimization of fluid management strategies in surgical intensive care settings.
3. Methods
3.1. Study Design and Setting
This was a prospective, intra-individual interventional clinical diagnostic study conducted in the Anesthesia and Surgical Intensive Care Unit at Viet Duc Hospital from October 2020 to October 2021.
3.2. Participants
Postoperative patients from the Department of Anesthesiology and Intensive Care at Viet Duc Hospital were enrolled between October 2020 and October 2021 if they met all of the following inclusion criteria: Age over 18 years; undergoing invasive mechanical ventilation; receiving at least one vasopressor; monitored using the PiCCO system; informed consent provided by family members. Exclusion criteria included: Lower limb fractures; severe traumatic brain injury; severe acute respiratory distress syndrome (ARDS) (P/F ratio ≤ 100 or PEEP > 10 cm H2O); decompensated heart failure or acute pulmonary edema; unresolved hemothorax or pneumothorax.
Based on previous studies Monnet et al. (4, 6), we estimated a standard deviation of approximately 0.3 L/min/m2 for ΔCI. Assuming a minimal clinically important difference of 0.2 L/min/m2, with α = 0.05 and power = 90%, the minimum required sample size for a paired comparison was 24 patients. To improve the precision of diagnostic accuracy estimates [e.g., area under the curve (AUC), sensitivity, specificity] and allow for exploratory subgroup analyses, we increased the final sample size to 31 patients.
3.3. Study Protocol
Following surgery, patients were prospectively enrolled and closely monitored throughout the early postoperative period in the surgical intensive care unit (SICU). Initial PiCCOTM (PULSION Medical Systems AG, Munich, Germany) measurements were performed, and baseline hemodynamic parameters (T0) were recorded. If the patient met the criteria for fluid administration, the next step was initiated. Otherwise, the patient continued to be monitored with PiCCO measurements every 6 hours or whenever hemodynamic instability occurred, such as increased heart rate or the need for higher vasopressor doses.
Following a 2-minute pre-oxygenation phase with FiO2 100%, patients underwent a 30-second EEO test, with Cardiac Index (CI) recorded at time point T1. After a 5-minute recovery period (T2), a 90-second PLR maneuver was performed, and CI was recorded at T3. Subsequently, a 500 mL crystalloid bolus (either 0.9% normal saline or Ringer lactate) was infused over 30 minutes, and post-infusion hemodynamic measurements were obtained at T4. Fluid responsiveness was defined as a ≥ 15% increase in ΔCI at T4 compared to baseline (T0) (7-9). Cardiac Index was assessed at five predefined time points: Baseline (T0), post-EEO (T1), post-recovery (T2), post-PLR (T3), and post-fluid challenge (T4), using the PiCCO™ system (PULSION Medical Systems AG, Munich, Germany). The CI at T0 and T4 was measured by transpulmonary thermodilution using three bolus injections of 20 mL cold 0.9% saline (< 8°C), and the average was recorded. The CI at T1, T2, and T3 was estimated using real-time arterial waveform analysis provided by the PiCCO pulse contour algorithm. All patients were mechanically ventilated in volume-controlled mode, with a tidal volume of 6 - 8 mL/kg and PEEP ≤ 10 cm H2O. For the PLR test, ΔCI was calculated as the relative percentage change in CI between T2 (post-recovery) and T3 (post-PLR), ΔCI (PLR) = [(CI at T3 - CI at T2)/CI at T2] × 100. For the EEO test, ΔCI was calculated as the percentage change from T0 (baseline) to T1 (post-EEO): ΔCI (EEO) = [(CI at T1 - CI at T0)/CI at T0] × 100.
A patient meets the criteria for fluid administration if they present with at least two clinical criteria and fulfill all PiCCO criteria, specifically, clinically: Fluid loss recorded (negative fluid balance); tachycardia > 100 bpm; hypotension or requiring high-dose vasopressors (mean arterial pressure (MAP) ≤ 65 mm Hg); low CVP < 8 cm H2O; oliguria (< 0.5 mL/kg/h); mottled skin; decreased central venous oxygen saturation (ScvO2) or mixed venous oxygen saturation (SvO2) ≤ 70%, lactate > 4 mmol/L. PiCCO: Extravascular Lung Water Index (ELWI) < 15 mL/kg; Global End-Diastolic Volume Index (GEDVI) < 900 mL/m2. The cutoff values for GEDVI (< 900 mL/m2) and EVLWI (< 15 mL/kg) were based on established thresholds from previous studies using PiCCO monitoring, aiming to ensure preload responsiveness while minimizing the risk of fluid overload.
3.4. Data Collection
Data were obtained by clinical examinations and review of medical records. Collected data encompassed:
3.4.1. Preoperative Variables
Age, gender, ASA classification, NYHA class, EuroSCORE II, medical history (including hypertension, cerebrovascular accident, diabetes, and history of alcohol abuse), glomerular filtration rate, preoperative serum albumin levels, and left ventricular ejection fraction.
3.4.2. Intraoperative Variables
Operative time and the number of surgical drainages.
3.4.3. Postoperative Variables
Duration of benzodiazepine and opioid use, length of mechanical ventilation, incidence of acute renal failure, and requirements for blood transfusion.
3.4.4. Definition of Fluid Responsiveness
Positive FR was defined as an increase in CI of ≥ 15% following fluid administration, in accordance with the criteria described by Marik (2).
3.5. Statistical Analysis
Data were analyzed using SPSS version 20.0. Normality of all continuous variables was assessed using the Shapiro-Wilk test before applying parametric or non-parametric statistical tests. Quantitative variables are presented as means ± standard deviations (SD) and were compared using the t-test for normally distributed data or the Mann-Whitney U test for non-normally distributed data. Qualitative variables are expressed as frequencies and percentages, with comparisons made using the chi-square test. A P-value of less than 0.05 was considered statistically significant. For hemodynamic comparisons, paired t-tests or Wilcoxon tests were used for pre- and post-test evaluations, while group comparisons were performed using independent t-tests. We used the Hanley-McNeil test to statistically compare the areas under the receiver operating characteristic (ROC) curves (AUCs) of EEO and PLR in predicting FR. The optimal cutoff value for each test was identified by maximizing the Youden Index (J), calculated as J = (Se + Sp - 1), where Se denotes sensitivity and Sp denotes specificity. In addition, the Bootstrap method was applied to estimate confidence intervals for the AUCs and to assess the statistical difference between the two tests.
Ethical approval was obtained from Hanoi Medical University (Decision No. 415 - 2021/QĐ-ĐHYHN) and from the leadership of the Department of Anesthesiology and Surgical Intensive Care at Viet Duc University Hospital.
4. Results
Among the 31 enrolled patients, 24 (77.4%) were classified as fluid responders. Compared to non-responders, responders had significantly higher SVV and pulse pressure variation (PPV), and a lower baseline CI. Gender distribution also differed significantly, with more male patients in the responder group (P = 0.0001). Other baseline characteristics, including age, vasopressor use, sequential organ failure assessment (SOFA) score, and CVP, were similar between groups (Table 1).
| Characteristic | Positive FR (n = 24) | Negative FR (n = 7) | P-Value |
|---|---|---|---|
| Age | 64.1 ± 19.4 | 55.2 ± 17.2 | 0.237 |
| Gender (male; %) | 74.2 | 25.8 | 0.0001 |
| Cardiovascular history (%) | 33.3 | 28.6 | 0.267 |
| SOFA score | 11.1 ± 0.5 | 10.9 ± 0.3 | 0.325 |
| Vasopressor | |||
| Norepinephrine (mcg/kg/min) | 0.52 ± 0.48 | 0.44 ± 0.35 | 0.702 |
| Epinephrine (mcg/kg/min) | 0.02 ± 0.06 | 0 | 0.43 |
| Dobutamine (mg/kg/min) | 1.39 ± 2.58 | 1.42 ± 2.43 | 0.973 |
| Lactate (mmol/L) | 4.1 ± 2.8 | 4.6 ± 5.5 | 0.769 |
| Hemodynamic parameters (T0) | |||
| Heart rate (BPM) | 107.4 ± 24.6 | 122.6 ± 24.2 | 0.325 |
| SAP (mmHg) | 119.2 ± 13.2 | 119.4 ± 13.4 | 0.160 |
| MAP (mmHg) | 78.2 ± 8.5 | 80.2 ± 14.9 | 0.972 |
| DAP (mmHg) | 57.7 ± 9.8 | 61.8 ± 16.3 | 0.650 |
| CI (L/min/m2) | 2.90 ± 0.37 | 3.32 ± 0.24 | 0.009 |
| SVV (%) | 14.4 ± 5.3 | 9 ± 1.3 | 0.013 |
| PPV (%) | 13.0 ± 4.7 | 7.1 ± 1.7 | 0.003 |
| GEDVI (mL/m2) | 660.7 ± 117.6 | 611.8 ± 67.0 | 0.305 |
| EVLWI (mL/kg) | 8.9 ± 2.4 | 10.1 ± 2.0 | 0.239 |
| SVRI (dynes sec cm-5m2) | 1884.3 ± 497.7 | 1734.9 ± 630.2 | 0.515 |
| CVP (cmH2O) | 11.6 ± 4.2 | 11.7 ± 7.4 | 0.515 |
Abbreviations: FR, fluid responsiveness; SAP, systolic arterial pressure; MAP, mean arterial pressure; DAP, diastolic arterial pressure; CI, Cardiac Index; SVV, stroke volume variation; PPV, pulse pressure variation; GEDVI, Global End-Diastolic Volume Index; EVLWI, Extravascular Lung Water Index; SVRI, Systemic Vascular Resistance Index; CVP, central venous pressure.
a Values are expressed as mean ± SD unless otherwise indicated.
b A P-value of ≤ 0.05 is considered statistically significant.
Neither the EEO nor PLR tests altered heart rate; however, both significantly affected MAP, systolic blood pressure (SBP), and CI. Both EEO and PLR increased the average CI, with a more pronounced rise observed in the fluid-responsive group (Table 2). No adverse events or unintended effects related to EEO or PLR were observed during the study.
| Parameter | EEO | PLR | ||||
|---|---|---|---|---|---|---|
| Baseline (T0) | Post EEO (T1) | P-Value | Post- recovery (T2) | Post PLR (T3) | P-Value | |
| HR (BPM) | ||||||
| Positive FR | 107.4 ± 24.6 | 107.8 ± 23.4 | > 0.05 | 107.5 ± 24.3 | 107.9 ± 22.6 | > 0.05 |
| Negative FR | 122.6 ± 24.2 | 121.7 ± 24.0 | > 0.05 | 121.3 ± 23.8 | 123.0 ± 0.7 | > 0.05 |
| SBP (mmHg) | ||||||
| Positive FR | 99.2 ± 13.2 | 105.7 ± 13.1 | < 0.05 | 101.4 ± 11.7 | 110.7 ± 12.0 | < 0.05 |
| Negative FR | 99.4 ± 13.4 | 107.1 ± 13.5 | < 0.05 | 102.0 ± 14.3 | 119.7 ± 16.3 | < 0.05 |
| DBP (mmHg) | ||||||
| Positive FR | 67.7 ± 9.7 | 71.9 ± 9.4 | < 0.05 | 70.0 ± 8.8 | 74.9 ± 10.4 | < 0.05 |
| Negative FR | 71.8 ± 16.3 | 75.1 ± 17.1 | < 0.05 | 71.6 ± 17.5 | 74.9 ± 14.6 | < 0.05 |
| CI (L/min/m2) | ||||||
| Positive FR | 2.9 ± 0.4 | 3.2 ± 0.4 | < 0.05 | 3.0 ± 0.4 | 3.5 ± 0.5 | < 0.05 |
| Negative FR | 3.3 ± 0.2 | 3.5 ± 0.2 | < 0.05 | 3.34 ± 0.2 | 3.7 ± 0.2 | < 0.05 |
Abbreviations: FR, fluid responsiveness; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; CI, Cardiac Index; EEO, end-expiratory occlusion; PLR, passive leg raising.
a Values are expressed as mean ± SD unless otherwise indicated.
b A P-value of ≤ 0.05 is considered statistically significant.
4.1. Predictive Ability of End-Expiratory Occlusion and Passive Leg Raising for Fluid Responsiveness Based on Change in Cardiac Index
When using changes in CI during testing to predict FR, the EEO test had a larger AUC compared to the PLR test (0.898 vs. 0.786). However, this difference was not statistically significant (P > 0.05). The optimal cutoff values were selected based on the maximum J (Se+Sp-1), which balances sensitivity and specificity. For PLR, the highest J value (0.658) corresponded to a ΔCI threshold of 13.4%. For EEO, the maximum J value (0.563) yielded a ΔCI threshold of 5.4% (Figure 1). Although both EEO and PLR demonstrated high predictive accuracy, the difference between their AUCs was not statistically significant (P = 0.06, Hanley-McNeil test).
5. Discussion
Our findings confirm that both the EEO test and PLR are effective in predicting FR in mechanically ventilated postoperative patients. While PLR induces more pronounced hemodynamic changes, EEO offers comparable predictive accuracy with higher sensitivity. This study was conducted on a diverse group of SICU patients, including those with traumatic shock, brain death, post-organ transplantation, and septic shock. Among the study population, 24 patients (77.4%) were fluid responsive, while 7 (22.6%) were non-responsive. This FR rate is consistent with that reported by Monnet et al. (6), who found a response rate of 52.3%. Variability across studies may be attributed to differences in patient populations, selection criteria, study designs, and methods used to assess FR. At baseline, there were no significant differences in heart rate, MAP, systolic or diastolic blood pressure, or preload indicators such as CVP and GEDVI between the responsive and non-responsive groups (P > 0.05). However, our findings suggest that patients with a lower CI are more likely to be fluid responsive, consistent with the observations of Messina et al. (5). Nevertheless, a definitive CI threshold for fluid resuscitation remains undetermined, as CI reflects current cardiac function rather than cardiac reserve. Therefore, CI alone is not considered a reliable predictor of FR.
5.1. Hemodynamic Changes During End-Expiratory Occlusion and Passive Leg Raising
Heart rate exhibited minimal changes during both EEO and PLR, likely due to its regulation by multiple factors beyond preload status, as noted by Monnet et al. (6). While MAP and diastolic blood pressure increased following these tests — possibly due to sympathetic stimulation or transient increases in cardiac output — these changes were not significantly different between responsive and non-responsive patients and, therefore, cannot be used as predictive markers. During EEO, fluid-responsive patients demonstrated a greater increase in CI compared to non-responsive patients (9.2% vs. 4.2%), though this was lower than the 12% ± 11% increase reported by Monnet et al. (4). Variations in CI response across studies may be due to differences in measurement techniques and patient selection. Notably, despite the 30-second apnea duration in our study, the CI change was not greater than that observed by Gavelli et al. (7) with a 15-second apnea period. In contrast, PLR resulted in a more pronounced CI change in fluid-responsive patients than in non-responsive patients. When comparing both tests within the same patients, PLR elicited a more significant CI response. This may be attributed to PLR mobilizing approximately 300 - 500 mL of blood, whereas CI changes during EEO are influenced by mechanical ventilation and lung function interactions.
5.2. Diagnostic Performance of End-Expiratory Occlusion and Passive Leg Raising
For EEO, a 5.3% change in CI was identified as the optimal cutoff, yielding a sensitivity of 95.8% and specificity of 85.7%, with an AUC of 0.898. These results align with those of Gavelli et al. (7), who reported an AUC of 0.91 and a sensitivity of 85%. A recent meta-analysis by Mulder et al. (8) reported pooled sensitivity and specificity of 87% and 90%, respectively, for the EEO test in predicting FR. The authors emphasized that EEO performance may vary with clinical context, monitoring method, and PEEP level. These findings support the robustness of EEO while highlighting the need for setting-specific adaptation. For PLR, Monnet et al. (6) found that a 10% CI increase optimally predicted FR, with a sensitivity of 91%, specificity of 100%, and an AUC of 0.937 (95% CI: 0.797 - 0.99). However, our study found a slightly lower predictive accuracy for PLR, potentially due to the heterogeneous nature of our patient population, which included individuals undergoing gastrointestinal surgery, dialysis, and those with arrhythmias — factors that could influence the reliability of PLR. When comparing EEO and PLR, PLR elicited more readily detectable hemodynamic changes. However, EEO had a slightly larger AUC, though the difference between the two ROC curves was not statistically significant (P = 0.66), consistent with the findings of Monnet et al. (4).
The significance of our study lies in its comparison of two widely used tests in a specific patient population — postoperative ICU patients. While previous studies have primarily focused on general ICU populations, postoperative patients present unique challenges due to factors such as surgical fluid shifts, anesthesia effects, and inflammatory responses. By demonstrating that both EEO and PLR remain effective in this subset of patients, our findings support their broader application in guiding fluid resuscitation strategies. Although dynamic indices have superior predictive value, static parameters such as CVP or GEDVI still play a role in identifying patients at risk of fluid overload and in decision thresholds. A combined approach using both dynamic and static variables might enhance safety and individualization of fluid management strategies.
An important clinical implication of our study lies in the complementary diagnostic profiles of EEO and PLR. The EEO demonstrated high sensitivity (95.8%), making it a useful screening tool to rule out non-responders, while PLR exhibited perfect specificity (100%), supporting its role in confirming true responders. No hemodynamic instability, arrhythmia, or ventilatory complications were observed during or after the tests. This suggests both EEO and PLR are safe in this population. These characteristics suggest that sequential or combined use of both tests may optimize fluid management. Incorporating EEO and PLR into standardized ICU protocols could improve individualized decision-making and help prevent unnecessary fluid administration.
In recent years, the integration of echocardiographic techniques into functional hemodynamic testing has expanded the clinical utility of both PLR and EEO. Monnet et al. demonstrated that transthoracic echocardiography (TTE) can be used to assess EEO-induced changes in cardiac output with high accuracy, even in critically ill patients lacking advanced monitoring systems (10). For PLR, Li et al. showed that changes in Vpeak measured by TTE during PLR predicted FR effectively in elderly postoperative patients, highlighting its applicability in this vulnerable subgroup (11). However, the cutoff values established in our study were derived using the PiCCO system and should not be directly extrapolated to TTE-derived parameters, given the fundamental differences in measurement techniques and signal dynamics.
5.3. Conclusions
Both EEO and PLR have strong predictive value for FR in postoperative patients. Although PLR resulted in greater hemodynamic changes than EEO, their predictive effectiveness remained comparable.
5.4. Limitations and Future Directions
Despite the promising findings, our study has several limitations. The small sample size limits statistical power and generalizability, preventing subgroup or multivariate analyses. Although we observed a significant gender imbalance, we could not assess the effects of age, sex, or surgical type on test performance. These factors should be explored in larger studies. Another limitation of our study is the gender imbalance between fluid responders and non-responders, with a predominance of male patients in the responder group. This disparity may limit the generalizability of our findings to a broader surgical population, particularly females. However, it is important to note that our study focused primarily on the physiological assessment of cardiac functional reserve using preload-modifying maneuvers (EEO and PLR), rather than on absolute outcome measures. These maneuvers are designed to evaluate intrinsic cardiac preload responsiveness and hemodynamic coherence within the same individual, and are therefore less likely to be significantly influenced by sex-related anatomical or hormonal differences. Nonetheless, future studies with more balanced gender representation are warranted to confirm these findings and explore potential sex-related variations in FR.
Furthermore, while the PiCCO system provides high-fidelity and continuous hemodynamic monitoring, its requirement for invasive arterial and central venous catheterization, along with the need for regular calibration, limits its applicability in resource-limited settings. These considerations should be kept in mind when interpreting and generalizing the study findings. Another consideration is the dynamic nature of FR itself. The FR is not a fixed characteristic but rather a transient physiological state influenced by evolving clinical conditions. Serial assessments and multimodal approaches may be necessary to optimize fluid therapy over time. Future research should explore how repeated testing or a combined use of EEO and PLR could improve decision-making in fluid management. Lastly, while our study focused on short-term hemodynamic changes, the long-term impact of fluid resuscitation guided by EEO and PLR remains an open question. Future studies should examine whether utilizing these tests to guide fluid administration improves clinical outcomes, including fewer ventilator days, reduced ICU mortality, and lower rates of postoperative complications. Furthermore, the integration of non-invasive hemodynamic monitoring with these maneuvers can also be explored. These findings support the integration of both tests into perioperative hemodynamic monitoring protocols to individualize fluid therapy.
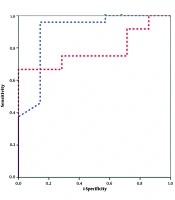
![Receiver operating characteristic (ROC) curves for end-expiratory occlusion (EEO) and passive leg raising (PLR) in predicting fluid responsiveness (FR); [EEO: Area under the curve (AUC) = 0.898, optimal cutoff = 5.3%; PLR: AUC = 0.786, optimal cutoff = 13.4%]. Receiver operating characteristic (ROC) curves for end-expiratory occlusion (EEO) and passive leg raising (PLR) in predicting fluid responsiveness (FR); [EEO: Area under the curve (AUC) = 0.898, optimal cutoff = 5.3%; PLR: AUC = 0.786, optimal cutoff = 13.4%].](https://services.brieflands.com/cdn/serve/3170c/352ed6548ccf738fc9524d64453e90bb48c953bd/jcma-10-3-161618-i001-preview.webp)