1. Background
Oral mucositis (OM) affects up to 50% of children undergoing chemotherapy, posing a significant barrier to nutritional intake, quality of life, and the continuity of cancer treatment (1, 2). The OM typically manifests within 3 - 15 days following chemotherapy and progresses from mucosal erythema to deep, painful ulcerations that may lead to secondary infections and systemic complications (3-5). The World Health Organization (WHO) classifies OM into five grades, ranging from 0 (no mucositis) to 4 (severe ulceration with complete inability to eat) (5).
Standard treatment approaches — such as topical anesthetics, antiseptic rinses, and systemic analgesics — often fail to provide adequate relief (6, 7). This therapeutic gap has prompted the exploration of novel topical agents that can deliver targeted analgesia with minimal systemic exposure.
Ketamine, a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, possesses anesthetic, analgesic, and mild anti-inflammatory properties (8). Topically administered ketamine may offer localized pain relief without significant systemic side effects. Although small-scale studies have demonstrated promise in both adult and pediatric populations (9-11), high-quality randomized trials in children remain limited.
2. Objectives
This study aims to evaluate the safety and analgesic efficacy of ketamine mouthwash for chemotherapy-induced OM in pediatric patients, utilizing a rigorous randomized, double-blind, placebo-controlled design. The primary objective was to assess the efficacy of ketamine mouthwash in reducing mucositis-related pain intensity compared with placebo. The secondary objectives were to evaluate the onset and duration of the analgesic effect and the safety profile.
3. Methods
This multicenter, randomized, double-blind, placebo-controlled, parallel-group trial was conducted at two tertiary pediatric oncology centers in Iran: Mofid Children’s Hospital (Tehran) and Ali Asghar Hospital (Zahedan). Ethical approval was obtained from the Shahid Beheshti Medical University Ethics Committee. Written informed consent was obtained from all guardians, and assent was obtained from children when appropriate. The study was prospectively registered (IRCT20201218049750N1).
Eligible participants were children aged 7 to 14 years diagnosed with WHO grade 3 or 4 OM following chemotherapy. Exclusion criteria included ketamine use within 48 hours prior to enrollment, known hypersensitivity to ketamine, and a history of acute psychosis. This was a multicenter, randomized, double-blind, placebo-controlled parallel trial with a 1:1 allocation ratio.
Participants were randomized in a 1:1 ratio using a permuted block design (block size = 8). Allocation concealment was ensured through sealed opaque envelopes. No protocol changes were made after trial commencement.
The intervention group received 5 mL of ketamine mouthwash (4 mg/mL) every 8 hours for 72 hours, with instructions to swish for 30 seconds and then spit. The placebo group received an identical-appearing normal saline solution. Blinding was maintained across participants and care providers.
1. Primary outcome: Pain intensity was measured on a 10-point Numeric Rating Scale (NRS) at baseline, 1 hour after each dose, and every 8 hours.
2. Secondary outcomes: Dietary intake (none, liquid, soft, regular), onset and duration of pain relief, and adverse event monitoring.
3.1. Sample Size
Based on pilot data, 40 patients (20 per group) provided 80% power to detect a 2-point difference in pain scores (α = 0.05). No interim analyses or stopping rules were planned.
3.2. Randomization and Blinding
Randomization was computer-generated with block size 4. Allocation was concealed by the pharmacist, who prepared sequentially numbered, identical syringes. Participants, caregivers, and investigators were blinded. Ketamine and placebo solutions were indistinguishable.
3.3. Statistical Analysis
Repeated measures analysis of variance (ANOVA) was used for pain score comparisons. Categorical data were analyzed using chi-square tests. P < 0.05 was considered statistically significant. No subgroup analyses were performed.
Statistical analysis was performed using SPSS v19. Continuous variables were compared using t-tests or Mann-Whitney U tests; categorical variables were analyzed using chi-square or Fisher’s exact tests. A P-value of < 0.05 was considered statistically significant.
4. Results
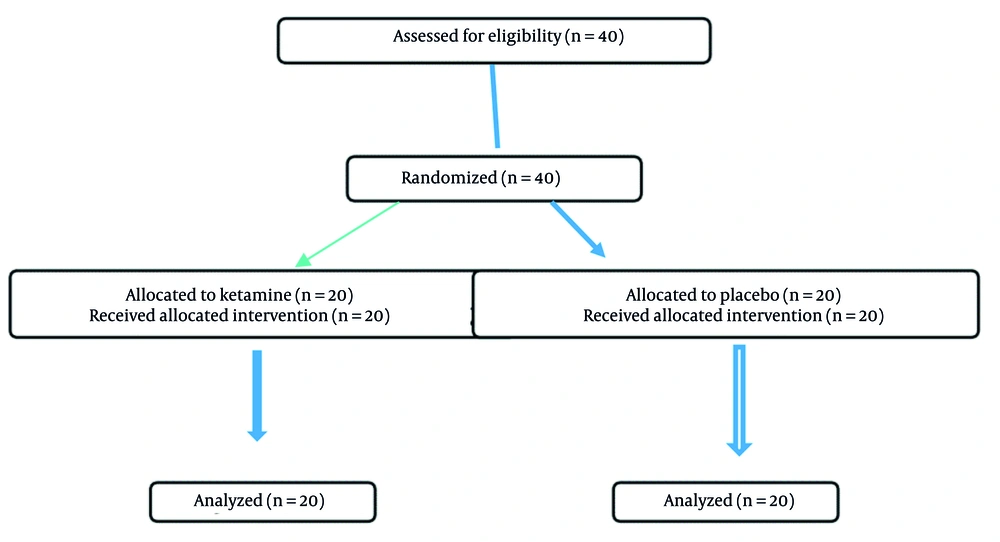
All 40 randomized participants completed the study. The ketamine group (n = 20; mean age: 9.9 ± 2.97 years) and the placebo group (n = 20; mean age: 8.9 ± 2.1 years) were comparable at baseline. Underlying malignancies included acute lymphoblastic leukemia (n = 19), acute myeloid leukemia (n = 11), lymphoma (n = 8), and germ cell tumors (n = 2). Demographic Information is presented in Table 1.
| Characteristics | Ketamine Group | Placebo Group |
|---|---|---|
| Age (y); mean ± SD | 9.9 ± 2.1 | 8.9 ± 1.9 |
| Sex | ||
| Male | 8 (40) | 12 (60) |
| Female | 12 (60) | 8 (40) |
| Diagnosis | ||
| ALL | 10 (50) | 9 (45) |
| AML | 5 (25) | 6 (30) |
| Lymphoma | 4 (20) | 4 (20) |
| Germ cell tumor | 1 (5) | 1 (5) |
a Values are expressed as No. (%) unless indicated.
All 40 randomized participants (20 per arm) were included in the analysis according to intention-to-treat principles. We clarified that 20 patients were allocated to the ketamine group and 20 to the placebo group. All participants received the assigned intervention and were analyzed for both the primary and secondary outcomes. A CONSORT-style flow diagram has been added Figure 1. Recruitment took place between January 2021 and December 2022, with follow-up completed in January 2023.
We reported the mean reduction in pain scores for each group with corresponding P-values. Confidence intervals (95%) for effect sizes have been added. As the outcomes were continuous (pain scores), binary effect sizes were not applicable. This has been clarified.
4.1. Pain Scores
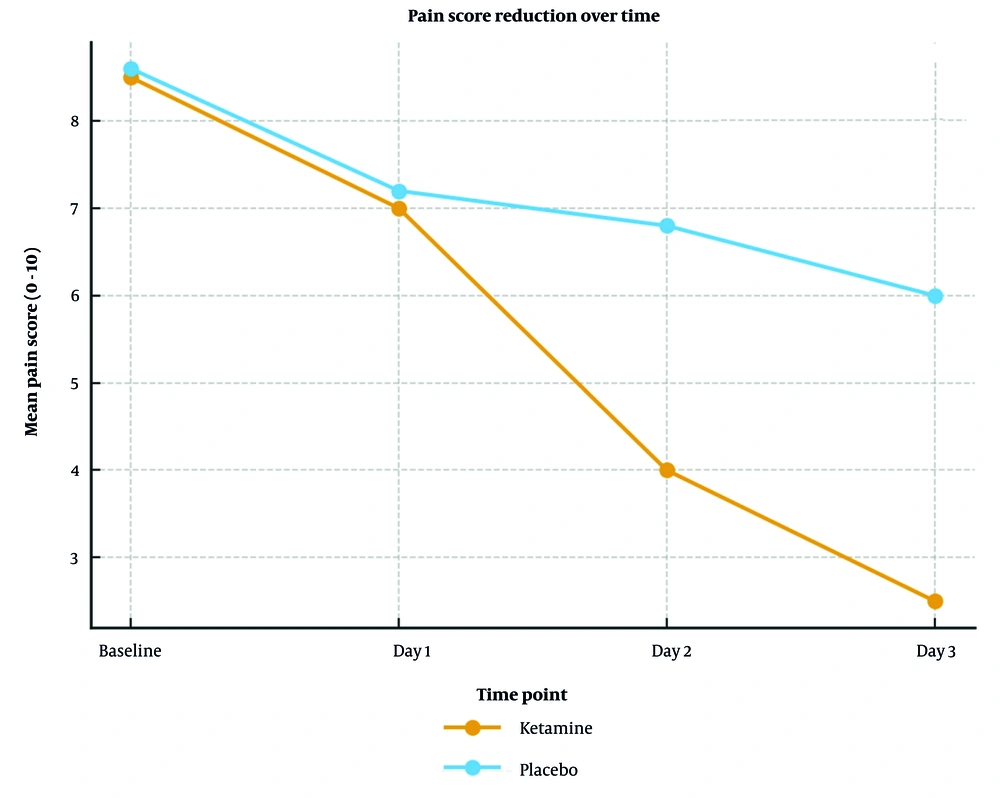
No difference was observed on day one (P = 1.0). However, by day two, pain scores had significantly declined in the ketamine group (mean reduction: 3.2 vs. 1.1; P < 0.0001) and continued to improve on day three (mean reduction: 4.1 vs. 1.9; P = 0.0003). Figure 2 is a line chart depicting the reduction in pain scores among patients. Patients in the ketamine group consistently reported pain relief within 15 minutes, lasting 2 - 3 hours.
4.2. Oral Intake
By day two, 70% of ketamine-treated patients resumed soft or regular diets compared to 20% in the placebo group (P < 0.001). By day three, this increased to 90% in the ketamine group, indicating superior functional recovery.
4.3. Adverse Events
No adverse events were reported in either group, and no patient required discontinuation or rescue therapy.
5. Discussion
This is the first double-blind, randomized, parallel trial to administer ketamine mouthwash to pediatric oncologic patients every eight hours over three days. This study assessed the efficacy and safety of ketamine mouthwash (4 mg/1 mL) in children with severe chemotherapy-induced mucositis above seven years of age. At the end of the second and third days, statistically significant pain reduction was reported by participants in the ketamine group compared to the placebo group (P < 0.001).
In contrast to our findings, the Satya Prakash research group reported that pain reduction did not differ significantly between their patients and the placebo group. However, they prescribed only a single dose of ketamine (9). Notably, they utilized a face scale for assessing pain, which is less suitable for patients in this age range. We used a numeric pain scale, which is the most reliable subjective pain assessment appropriate for our patients’ age group. Shillingburg et al. indicated that 30 adult oncologic patients who received ketamine solution at a concentration of 20 mg/5 mL experienced significant reductions in mucositis pain. Their study design was open-label and therefore more susceptible to bias compared to our trial, which was randomized, double-blinded, and placebo-controlled (10). Slatkin and Rhiner described a case of a 32-year-old female with tongue squamous carcinoma who suffered from severe and refractory pain; ketamine mouthwash (20 mg/5 mL) was administered, resulting in significant pain reduction lasting for one hour, and she was discharged with this medication to be used every 3 hours (11). According to a retrospective study, pain reduction was observed in 5 out of 8 patients (12). Similar results were seen in a trial by Saenz et al.; their study found ketamine mouthwash to be effective in treating orofacial pain in adult cancer patients. When combined with oral transmucosal fentanyl citrate, the analgesic efficacy reached 94.1%. However, some transient side effects were associated with the ketamine mouthwash (13). That study was retrospective, and the efficacy of ketamine mouthwash was not analyzable; in contrast, our trial was a prospective, randomized, controlled study with an appropriate methodological design. Our study limitations include the limited sample size, although the impact was mitigated by the rigorous study design.
This trial demonstrates that ketamine mouthwash (4 mg/mL) administered every 8 hours for 72 hours is safe, well-tolerated, and effective in reducing pain and improving oral intake in pediatric patients with severe chemotherapy-induced mucositis.
Our findings are consistent with adult case series and early pediatric reports suggesting a benefit of topical ketamine (9-11), but differ in the strength of design: A double-blind, placebo-controlled methodology utilizing self-reported numeric pain scores. This enhances reliability and reduces observer bias, which affected previous open-label studies. Our results also align with case reports (11) and small series (12), and are comparable to retrospective adult experiences (13). Furthermore, our findings contribute to the body of evidence on interventions such as doxepin rinse (14), herbal mouthwashes (15), and sucralfate rinse (16). These findings support ketamine mouthwash as a promising analgesic option in pediatric oncology.
The analgesic effect was rapid (onset within 15 minutes) and sustained over multiple doses, underscoring ketamine’s value in localized pain management. The significant improvement in oral intake highlights functional recovery, an often overlooked but crucial endpoint in mucositis trials.
In light of emerging literature on ketamine’s evolving role in pain management, including concerns regarding systemic safety and optimal dosing strategies (12, 13), our study provides valuable pediatric-specific data demonstrating localized benefit without systemic complications.
5.1. Conclusions
Ketamine mouthwash (4 mg/mL) appears to be a safe and effective intervention for severe OM in pediatric oncology patients. Its rapid analgesic onset, lack of systemic toxicity, and facilitation of oral intake support its role as a valuable supportive care option during chemotherapy.
5.2. Limitations
The small sample size and short follow-up period limit generalizability. Larger multicenter studies are needed to determine optimal dosing frequency, long-term safety, and effects across different cancer protocols.