1. Background
Cystic fibrosis (CF) is an autosomal recessive disorder that results from mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (1, 2). It mainly affects the lungs and pancreas; however, it also affects the upper airways, liver, intestines, and reproductive organs (3). Although CF is generally a multi-organ disorder (4, 5), progressive lung involvement is the main cause of morbidity and mortality in most patients (6).
Previous research has shown that pulmonary injury in patients with CF begins during infancy or early childhood (7). Spirometry is regarded as the gold standard for assessing lung function in children older than 6 years and in adults (8). However, performing spirometry may not be practical during acute exacerbations, and children with more severe impairment may be unable to complete spirometry according to standard guidelines, as it requires forced expiratory efforts. Moreover, studies have demonstrated that spirometry may lack sufficient sensitivity for detecting mild to moderate lung damage in children with CF (9-11).
Impulse oscillometry (IOS), also referred to as the forced oscillation technique (FOT), employs sound waves and has been utilized in preschool-aged children and in other contexts where forced exhalation cannot be performed (12). The IOS has been applied to evaluate treatment response, bronchodilator responsiveness, methacholine challenge testing, and long-term monitoring in pediatric asthma. Consequently, IOS may have a valuable role in the routine evaluation of children with CF (13).
Studies have reported the correlation between IOS parameters and spirometric parameters in children with asthma. Also, IOS is sensitive for diagnosing airway obstruction in patients with normal spirometry. However, limited information is available in CF patients (14). Recent evidence suggests that IOS may serve as an alternative assessment in patients who are unable to undergo spirometry. Accordingly, in view of its potential advantages but also the inconsistent findings regarding IOS applicability in CF, additional research is warranted (12, 15).
2. Objectives
Given the scarcity of related studies worldwide, the present investigation was designed to examine, for the first time in Iran, the relationship between IOS indices and spirometric parameters in children with CF.
3. Methods
In this cross-sectional study, 51 children with CF aged 6 to 18 years who were referred to Mofid Children's Hospital, Tehran, in 2022 and 2023 were enrolled. The diagnosis of CF was confirmed by clinical symptoms, sweat chloride level ≥ 60 mmol/L, and gene mutation analysis. The inclusion criteria were CF patients between 6 and 18 years of age who were able to perform forced expiration according to the American Thoracic Society (ATS) guidelines and whose parents provided consent to participate in the study. Patients were excluded if they were in a severe stage of CF, had any lung disease other than CF, had evidence of severe respiratory infection in the last one month, were unable to perform pulmonary function tests, refused to perform the spirometry test at Mofid Hospital, or had incomplete records. The study protocol received approval from the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1402.420).
3.1. Spirometry
Based on the ATS guidelines, spirometry was performed using the MasterScreen device in cooperative children aged ≥ 6 years in the seated position (16). Forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC, and forced expiratory volume 25 - 75 (FEV25-75) were measured using a Jaeger Masterlab pneumotachograph (Erich Jaeger GmbH) and reported both as absolute values and as percentages of the predicted values (12).
3.2. Impulse Oscillometry
Additionally, for each patient, the IOS test was performed using a mouthpiece while the patient was seated, with a nose clip applied and cheeks supported. The IOS-related parameters were measured by analyzing the pressure-flow relationship following the superimposition of short pressure impulses on tidal airflow, including resistance of the entire respiratory system (Z), resistance of airways and lung tissue (R), and reactance (X) between 5 and 20 Hz.
3.3. Statistical Analysis
Data were analyzed using SPSS software, Version 22. The Kolmogorov-Smirnov and Shapiro-Wilk tests were applied to assess the normality of variable distributions. Quantitative variables are presented as mean ± standard deviation (SD), and qualitative variables as number (percentage). Pearson’s correlation test was employed to examine associations between variables. Multiple linear regression analysis was used to explore the relationships between IOS parameters [resistance at 5 Hz (R5), resistance at 20 Hz (R20), R5-R20, reactance at 5 Hz (X5), area of reactance (AX)] and Spirometric indices (FEV1, FEV1/FVC, FEF25-75, and FVC), with age (continuous) and gender (categorical) included as covariates. All statistical procedures were performed using SPSS Version 22. A P-value < 0.05 was considered indicative of statistical significance.
4. Results
The mean ± SD age of the study population was 12.06 ± 4.31 years, with a male to female ratio of 1.4:1. The mean ± SD sweat test result was 81.73 ± 10.86 mmol/L. The mean ± SD values for FEV1 and FVC were 75.77 ± 33.14 and 90.22 ± 36.79 percentages, respectively. Additionally, the mean ± SD FEV25-75 was 55.14 ± 34.42 l/s. The mean ± SD FEV1/FVC was 79.76 ± 14.03. The mean ± SD R5 and R20 were 107.94 ± 44.57 and 93.37 ± 34.35, respectively. Detailed data for IOS parameters are provided in Table 1.
| Variables | Values |
|---|---|
| Sweat test (mmol/L) | 81.73 ± 10.86 |
| FEV1 (%) | 75.77 ± 33.14 |
| FVC (%) | 90.22 ± 36.79 |
| FEV25-75 (L/S) | 55.14 ± 34.42 |
| FEV1/FVC | 79.76 ± 14.03 |
| R5 | 107.94 ± 44.57 |
| R20 | 93.37 ± 34.35 |
| R5-R20 | 168.99 ± 257.26 |
| X5 | -75.27 ± 835.73 |
| AX | 4.83 ± 10.41 |
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV25-75, forced expiratory volume 25 - 75; R5, resistance at 5 Hz; R20, resistance at 20 Hz; X5, reactance at 5 Hz; AX, area of reactance.
a Values are expressed as mean ± standard deviation (SD).
Considering that both IOS and spirometry parameters are quantitative variables, correlation tests were used to measure the relationships. A modest negative correlation was observed between R5 and FEV1 (R = -0.298, P = 0.034), suggesting a limited but statistically significant association. A stronger negative correlation was observed between the R5 Index and FEV25-75 (R = -0.358, P = 0.01). There was a significant inverse relationship between the R5 parameter and FEV1/FVC (R = -0.474, P = 0.0001). The highest correlation value was observed between the R20 parameter and FEV1/FVC (R = -0.479, P = 0.0001). In addition, R20 had a significant negative relationship with FEV25-75 (R = -0.385, P = 0.005). Other IOS parameters had no remarkable relationship with spirometry parameters (P > 0.05). More details regarding correlations between IOS and spirometry parameters are provided in Table 2.
| Variables | Sweat Test | FEV1 | FVC | FEV25-75 | FEV1/FVC |
|---|---|---|---|---|---|
| R5 | |||||
| R | 0.187 | -0.298 | -0.16 | -0.358 | -0.474 |
| P-value | 0.18 | 0.034 | 0.24 | 0.01 | 0.0001 |
| R20 | |||||
| R | 0.15 | -0.27 | -0.15 | -0.385 | -0.479 |
| P-value | 0.27 | 0.055 | 0.26 | 0.005 | 0.0001 |
| R5-R20 | |||||
| R | 0.15 | 0.012 | 0.053 | -0.042 | -0.073 |
| P-value | 0.28 | 0.934 | 0.71 | 0.76 | 0.609 |
| X5 | |||||
| R | -0.249 | 0.190 | 0.204 | 0.18 | 0.183 |
| P-value | 0.078 | 0.18 | 0.15 | 0.14 | 0.20 |
| AX | |||||
| R | -0.085 | -0.036 | -0.103 | 0.039 | 0.115 |
| P-value | 0.55 | 0.802 | 0.47 | 0.78 | 0.42 |
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV25-75, forced expiratory volume 25 - 75; R5, resistance at 5 Hz; R20, resistance at 20 Hz; X5, reactance at 5 Hz; AX, area of reactance.
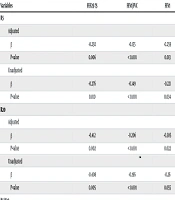
To account for potential confounding by age and gender, multiple linear regression analyses were conducted. The adjusted results (Table 3) confirmed significant negative relationships between R5 and FEF25-75 (β = -0.292, P = 0.006), FEV1/FVC (β = -0.155, P < 0.001), and FEV1 (β = -0.259, P = 0.013), consistent with unadjusted findings (R = -0.358, P = 0.010; R = -0.474, P < 0.001; R = -0.298, P = 0.034, respectively). Similarly, R20 showed significant negative relationships with FEF25-75 (β = -0.412, P = 0.002), FEV1/FVC (β = -0.206, P < 0.001), and FEV1 (β = -0.305, P = 0.022), aligning with unadjusted results (R = -0.385, P = 0.005; R = -0.479, P < 0.001; R = -0.270, P = 0.055, respectively). The modest correlation between R5 and FEV1 (R = -0.298, P = 0.034) suggests a limited but statistically significant association. No significant relationships were observed for R5-R20, X5, or AX with any spirometry parameters (P > 0.05). The relationship between R5 or R20 and FVC remained non-significant (adjusted P = 0.108 and P = 0.109, respectively).
| Variables | FEF25-75 | FEV1/FVC | FEV1 | FVC |
|---|---|---|---|---|
| R5 | ||||
| Adjusted | ||||
| β | -0.292 | -0.155 | -0.259 | -0.181 |
| P-value | 0.006 | < 0.001 | 0.013 | 0.108 |
| Unadjusted | ||||
| β | -0.276 | -0.149 | -0.221 | -0.136 |
| P-value | 0.010 | < 0.001 | 0.034 | 0.249 |
| R20 | ||||
| Adjusted | ||||
| β | -0.412 | -0.206 | -0.305 | -0.230 |
| P-value | 0.002 | < 0.001 | 0.022 | 0.109 |
| Unadjusted | ||||
| β | -0.408 | -0.196 | -0.26 | -0.170 |
| P-value | 0.005 | < 0.001 | 0.055 | 0.266 |
| R5-R20 | ||||
| Adjusted | ||||
| β | -0.004 | -0.003 | 0.003 | 0.010 |
| P-value | 0.844 | 0.680 | 0.855 | 0.599 |
| Unadjusted | ||||
| β | -0.006 | -0.004 | 0.002 | 0.008 |
| P-value | 0.769 | 0.609 | 0.93 | 0.713 |
| X5 | ||||
| Adjusted | ||||
| β | 0.001 | -0.001 | 0.003 | 0.002 |
| P-value | 0.834 | 0.766 | 0.662 | 0.760 |
| Unadjusted | ||||
| β | 0.008 | 0.003 | 0.008 | 0.009 |
| P-value | 0.184 | 0.200 | 0.181 | 0.151 |
| ALX | ||||
| Adjusted | ||||
| β | 0.040 | 0.104 | -0.146 | -0.393 |
| P-value | 0.930 | 0.555 | 0.742 | 0.404 |
| Unadjusted | ||||
| β | 0.128 | 0.155 | -0.115 | -0.632 |
| P-value | 0.788 | 0.422 | 0.802 | 0.474 |
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; R5, resistance at 5 Hz; R20, resistance at 20 Hz; X5, reactance at 5 Hz.
5. Discussion
Pulmonary function assessments play a crucial role in diagnosing and monitoring airway disease in CF. Conventional spirometry, which depends on repeated forced expiratory maneuvers, is regarded as the standard approach. In contrast, IOS is a non-invasive technique that requires only minimal patient cooperation (11, 17).
The present study was carried out to examine the association between IOS parameters and spirometric measures in patients with CF. Our findings showed that there was a slight negative correlation between the R5 parameter and FEV1. There was a stronger and inverse correlation between the R5 Index and FEV25-75. Also, R20 had a significant negative relationship with FEV25-75. The prospective study by Raj et al. in patients with CF demonstrated a moderate, statistically significant negative correlation between Spirometric indices [FEV1, FVC, and peak expiratory flow rate (PEFR)] and IOS variables [impedance at 5 Hz (Z5) and R5]. In addition, X5 showed a significant but weak positive correlation with FEV1 and FVC, whereas RF exhibited a significant yet weak negative correlation with FEV1 and FVC (12). These findings align with our results, supporting the notion that IOS can serve as an alternative pulmonary function test in individuals with CF, particularly in those who are unable to perform spirometry.
There are a limited number of studies in patients with CF that have aimed to assess and monitor lung function with IOS. The relationship between spirometry and IOS outcomes in children with CF is inconsistent (18). Nikkhah et al. found a correlation between R5, R20, and X5 with FEV1 in patients with asthma and also reported a significant correlation between R5 and FEV1 in patients with chronic obstructive pulmonary disease (COPD) (19). These findings, together with our own results, emphasize that IOS may serve as a suitable alternative to spirometry for identifying obstructive lung disease, whether in asthma, COPD, or CF.
Kanik et al., in a study that compared clinical severity scores and conventional spirometry with IOS measurements and thoracic high-resolution computed tomography findings in children with CF, reported that individuals with FEV1 values below 80% showed significantly elevated resistance parameters (R5 and R10) and markedly reduced X5 values on IOS (18). In the study of Ozturk et al., a significant relationship between parameters of spirometry test and IOS was observed in 70 CF patients (15). Ren et al. used spirometry and FOT to assess respiratory exacerbations in hospitalized CF children. They showed a notable relationship between changes in FEV1 and X5 before and after treatment (20).
In a study by Lundberg et al., the results of spirometry and IOS were considerably correlated in premature children, with the strongest correlation belonging to the FEV and the AX. In their study, spirometry and IOS parameters were significantly correlated in preterm infants (21). The results of these studies were in line with our findings.
Moreau et al. evaluated the correlation between IOS parameters and spirometry tests in 30 CF patients. The X5 had a positive correlation with spirometry parameters. When the percentage predicted values were considered, the correlations were non-significant and weak (14). Our findings were inconsistent with the findings of Moreau's study. The difference in the sample size and the type of oscillometry and spirometry device may be the cause of the contradiction in the results.
Blin et al. evaluated IOS and spirometry parameters in adults with CF during both stable periods and acute exacerbation episodes. Although the performance of both spirometry and IOS parameters (X5, R5-R20, and AX) to discriminate acute exacerbation from stable state in CF was poor, they concluded that variation of IOS indices (X5, R5-R20, AX) in comparison with a personal reference value may be helpful to diagnose exacerbations of chronic airways disease, particularly when spirometry is unreliable or uncomfortable (22).
In the 2025 study by Heydarazad Zadeh et al., which examined the relationship between spirometric parameters and IOS in patients newly diagnosed with asthma, the findings indicated that FVC, FEV1/FVC, and FEF25-75 demonstrated a notable positive correlation with all IOS indices except for AX (23). Our findings are inconsistent with the results of their study. The discrepancy may be due to differences in sample size, different diseases in the two studies, and testing errors.
Postek et al. noted that oscillometry correlates with spirometry, which is considered the gold standard. For obstructive conditions such as asthma, IOS appears to be a useful diagnostic modality. In contrast, for CF, its role is more appropriately considered as a complementary assessment. Moreover, according to the existing evidence, it should be noted that oscillometry cannot substitute spirometry for monitoring the respiratory status of children and adolescents with CF (24).
One of the limitations of the study is the small sample size of patients with CF due to the single-center and cross-sectional nature of the study, which reduces the generalizability of the findings.
5.1. Conclusions
Given the correlations observed between certain spirometric and oscillometric parameters, IOS can be utilized when patients are unable to perform spirometry, in preschool children, and in other situations where forced exhalation is not feasible. One of the main strengths of this study is that both spirometry and oscillometry were carried out by a pulmonologist with high precision, and all patients received thorough instruction before testing. It is recommended that further multicenter studies with larger sample sizes be conducted to more comprehensively evaluate the relationship between IOS and spirometry parameters in children with CF.