1. Context
As the global population ages, cognitive decline has become a significant public health concern, affecting millions of older adults worldwide (1, 2). Age-related cognitive decline manifests in various domains, including memory, executive function, speech, language, and processing speed, ultimately impacting daily functioning, communication, and quality of life (3, 4). While crystallized cognition, such as vocabulary and acquired knowledge, tends to remain relatively stable, declines in fluid cognitive abilities, such as processing speed, attention, and executive functioning, pose challenges for healthy aging (5). Traditional pharmacological interventions have often failed to provide satisfactory results and may carry undesirable side effects (6). Consequently, there is growing interest in non-invasive brain stimulation techniques like transcranial direct current stimulation (tDCS) as promising alternatives to support cognitive health in older adults in rehabilitation fields (7-9).
The tDCS delivers a low electrical current to targeted brain regions via scalp electrodes, modulating neuronal excitability and potentially enhancing cognitive functions (10-14). Research has shown that tDCS can improve cognitive domains such as working memory and executive function by facilitating synaptic plasticity and neural connectivity (15, 16). Moreover, tDCS has demonstrated potential benefits in episodic memory for both healthy older adults and individuals with mild cognitive impairments (17, 18). These cognitive improvements are thought to arise from tDCS-induced changes in cortical excitability, which may promote more efficient neural processing and compensatory network activity in the aging brain (19).
Despite these promising findings, the efficacy of tDCS remains inconsistent across studies, largely due to variability in stimulation parameters, including current intensity, electrode placement, intervention duration, and individual differences among participants (2, 20). Factors such as baseline cognitive status, age, sex, and even genetic predispositions may influence individual responsiveness to tDCS, further complicating the interpretation of results (8, 21-26). Additionally, the durability of cognitive gains and the optimal timing and frequency of stimulation sessions are not yet fully understood (27). Meta-analyses report mixed findings, with some studies highlighting immediate cognitive benefits while others suggest limited or no long-term effects, underscoring the need to identify optimal protocols to maximize cognitive benefits (28, 29).
Despite increasing research on tDCS in aging populations, critical gaps remain. Notably, there is a lack of standardized stimulation protocols tailored specifically for cognitively intact older adults, leading to substantial methodological heterogeneity across studies. This variability, including differences in current intensity, session number, electrode placement, and outcome assessment, hinders clear conclusions about tDCS efficacy. Moreover, individual differences such as baseline cognitive status, age-related neurophysiological changes, and other participant characteristics are seldom systematically addressed, contributing to inconsistent findings. Consequently, there is a pressing need for a comprehensive synthesis that identifies optimal stimulation parameters and evaluates the methodological quality of existing randomized controlled trials (RCTs) to guide future clinical and research applications.
Given these complexities, there is a pressing need for the systematic evaluation and refinement of tDCS protocols tailored specifically to healthy older adults. This systematic review aims to synthesize current evidence on tDCS interventions targeting cognitive functions in this population, with a particular focus on optimizing stimulation parameters and understanding factors that influence individual responsiveness. By evaluating RCTs published between 2015 and 2025, this study seeks to provide practical insights for refining tDCS protocols to enhance cognitive aging and inform future rehabilitation strategies effectively. Ultimately, a clearer understanding of how to best implement tDCS could pave the way for safer, more effective, and personalized approaches to maintaining cognitive health in older adulthood.
Cognitive decline among older adults is a growing global health challenge with significant personal and societal burdens. Existing treatments are limited, prompting a need for innovative non-pharmacological interventions. Identifying effective and safe interventions to promote cognitive health in aging populations is essential for maintaining quality of life and functional independence. A systematic review of RCTs provides the highest level of evidence by rigorously evaluating the efficacy of tDCS across diverse study designs and participant populations, ensuring reliable conclusions.
2. Objectives
This systematic review aims to comprehensively assess the effectiveness of tDCS for cognitive enhancement in healthy older adults by identifying optimal stimulation protocols, critically appraising study quality, and synthesizing evidence on individual variability in responsiveness.
3. Methods
3.1. Study Design
This systematic review included a comprehensive literature search of PubMed, Google Scholar, Web of Science, and Scopus databases to identify English-language RCTs published between 2015 and 2025 assessing tDCS effects on cognitive outcomes in healthy older adults aged 65 and above. Screening and study selection followed established PRISMA guidelines. All procedures adhered to the ethical guidelines and regulations of the Tabriz University of Medical Sciences Ethics Committee, as outlined in the ethics code IR.TBZMED.REC.1403.708.
3.2. Search Strategy
A comprehensive search was performed across four electronic databases: PubMed, Google Scholar, Web of Science, and Scopus. The search combined keywords related to the population, intervention, control, and outcomes, including ("older adults" OR "elderly" OR "aging") AND ("transcranial direct current stimulation" OR "tDCS" OR "non-invasive brain stimulation") AND ("sham stimulation" OR "placebo" OR "control group") AND ("cognitive function" OR "cognitive performance" OR "memory" OR "executive function"). The review process adhered to the PRISMA guidelines (30).
The search terms and inclusion/exclusion criteria were developed and guided by the PICO framework to ensure a focused and systematic retrieval of the literature. Our keyword selection specifically reflected each PICO component as follows: Terms such as "older adults" and "aging" were used to capture studies involving the target demographic of healthy older individuals (population). Keywords included "transcranial direct current stimulation" and its abbreviation "tDCS" to identify relevant non-invasive brain stimulation interventions (intervention). Search terms such as "sham stimulation" and "control" were incorporated to distinguish between active intervention and control groups (comparison). Keywords related to cognitive health, including "cognitive function", "memory", and other cognitive performance indicators, were included to target relevant study outcomes (outcomes).
3.3. Methodological Evaluation
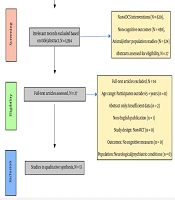
Titles retrieved from each database were exported as RIS files and imported into Covidence software for systematic screening (30). Two independent reviewers screened abstracts and excluded irrelevant studies. In cases of disagreement, a third reviewer was consulted to reach a consensus. Duplicate records were identified and removed within Covidence (30). Studies that passed abstract screening were assessed via full-text review based on predefined inclusion and exclusion criteria, as presented in Box 1. The screening process is outlined in the PRISMA flow diagram, as shown in Figure 1.
| Inclusion |
| The tDCS as the stimulation technique for intervention |
| Randomized or pseudo- (active and sham) with pre-RCTs and post-assessment |
| Cognition as the primary measured outcome |
| Age ≥ 65 |
| Written in English |
| Cognitively intact or cognitively normal participants |
| Exclusion |
| Observational studies, review articles, published abstracts, and case studies |
| Using tDCS in combination with other stimulation techniques |
| Diagnosis of neurological or psychiatric diagnosis or impairments, or major neurocognitive disorder such as mild cognitive impairment or dementia |
Abbreviations: tDCS, transcranial direct current stimulation; RCTs, randomized controlled trial.
3.4. Eligibility Criteria and Data Extraction
The included studies were randomized or pseudo-RCTs published in English, featuring pre- and post-intervention cognitive assessments. Studies involving healthy older adults without neurological or psychiatric disorders were selected. Exclusion criteria included observational studies, reviews, abstracts, case reports, qualitative studies, protocols, and studies involving participants with mild cognitive impairment or dementia. Interventions had to involve tDCS alone (without pharmacological or combined brain stimulation treatments) compared to sham controls, regardless of electrode montage. The primary outcome measure was cognitive performance. Data extraction focused on participant demographics, tDCS parameters (such as intensity, duration, and electrode placement), cognitive domains assessed, and effect sizes.
3.5. Risk of Bias
Data extraction encompassed participant demographics, detailed tDCS parameters (including current intensity, number of sessions, stimulation duration, and electrode montage), and cognitive performance measures across various domains. Methodological quality and risk of bias of the included RCTs were independently evaluated by two reviewers using the Cochrane Risk of Bias Tool version 2 (RoB 2) (31). Discrepancies were resolved by discussion or by consulting a third reviewer; detailed RoB 2 ratings for each study are presented in Table 1. The tool evaluates bias across five domains: Bias arising from the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result.
| Author(s) | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of Outcome | Selection of Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Antonenko, et al. (32) | Low risk | Some concerns | Some concerns | Low risk | Low risk | Some concerns |
| Au, et al. (33) | High risk | High risk | Low risk | Some concerns | Some concerns | High risk |
| Boutzoukas, et al. (34) | Low risk | Some concerns | Low risk | Low risk | Low risk | Low risk |
| Hardcastle, et al. (35) | High risk | High risk | Some concerns | Low risk | Some concerns | High risk |
| Hausman, et al. (36) | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Klink, et al. (37) | High risk | High risk | Low risk | Some concerns | Some concerns | High risk |
| Krebs, et al. (38) | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Kulzow, et al. (39) | High risk | High risk | Some concerns | Low risk | Some concerns | Low risk |
| Manor, et al.(40) | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Mehrdadian, et al. (41) | High risk | High risk | Low risk | Low risk | Some concerns | High risk |
| Melendez, et al. (42) | Low risk | Some concerns | Low risk | Some concerns | Low risk | Some concerns |
| Cruz Gonzalez, et al. (43) | High risk | High risk | Low risk | Low risk | Some concerns | High risk |
| Satorres, et al. (44) | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
Study screening and selection were conducted independently by two reviewers using Covidence software. Titles and abstracts were initially screened for relevance, followed by full-text review of potentially eligible studies. Disagreements between reviewers at any stage were resolved through discussion and consensus; when consensus was not reached, a third reviewer adjudicated. Inter-rater reliability was assessed using Cohen’s Kappa statistic, yielding a value of 0.82, indicating strong agreement between reviewers. The studies were critically appraised and are detailed in the Results section, with summaries provided in Tables 1 and 2.
| PRISMA Item | Description/How Addressed in Manuscript | Location in Manuscript (Page/Section) |
|---|---|---|
| Title | Identifies the report as a systematic review | Page 1, title |
| Abstract | Structured abstract includes objectives, methods (including RoB tool), results with key numeric findings | Abstract (page 2) |
| Introduction | ||
| Rationale | Background on tDCS and cognitive aging, highlighting inconsistencies | Introduction, paragraphs 1 - 3 (pages 3 - 4) |
| Objectives | Explicit research gap and clear study aims stated | Introduction, paragraph 4 (page 4) |
| Methods | ||
| Eligibility criteria | Inclusion/exclusion criteria for studies explained | Methods, eligibility criteria (page 5) |
| Information sources | Databases searched and date ranges specified | Methods, information sources (page 5) |
| Search strategy | Keywords, search strings used | Methods, search strategy (page 5) |
| Selection process | Screening and inclusion process described (PRISMA flowchart) | Methods, study selection (page 5), Figure 1 |
| Data collection process | Data extraction protocols, independent reviewers | Methods, data extraction (page 6) |
| Risk of bias assessment | Use of RoB 2, dual independent review | Methods, quality assessment (page 6) |
| Effect measures | Main outcome measures (SMD, CIs) | Methods, data synthesis (page 6) |
| Results | ||
| Study selection | Number of studies screened, excluded, included | Results, study selection (page 7), Figure 1 |
| Study characteristics | Description of participant demographics, tDCS parameters | Results, study characteristics (pages 7 - 8) |
| Risk of bias within studies | Summary of RoB 2 evaluation, overall bias risk per study | Results, risk of bias (page 8), Tables 1 and 2 |
| Results of individual studies | Key findings per study, including effect sizes | Results (page 8) |
| Synthesis of results | Pooled SMD = 0.35 (95% CI: 0.12 to 0.58) for working memory | Results, meta-analysis (page 9), abstract |
| Discussion | ||
| Interpretation | Interpretation of findings, limitations and implications discussed | Discussion section (pages 10 - 12) |
| Other information | ||
| Registration | Protocol not prospectively registered; statement included | Methods (page 5) |
| Support | Funding and conflict of interest statement | Conflict of interest (page 13) |
Abbreviations: RoB 2, Cochrane Risk of Bias Tool version 2; SMD, standardized mean difference; CI, confidence interval; tDCS, transcranial direct current stimulation.
Quantitative synthesis revealed a pooled standardized mean difference (SMD) of 0.35 [95% confidence interval (CI): 0.12 to 0.58], favoring active tDCS over sham in improving working memory performance following interventions of at least ten sessions at 2 mA intensity (31). However, effect sizes varied across studies, with some reporting non-significant or null results. Executive function and verbal fluency showed smaller and less consistent effect sizes, highlighting variability influenced by stimulation parameters and participant characteristics.
4. Results
4.1. Study Selection
The comprehensive literature search and manual screening identified a total of 2,359 studies. After removing 938 duplicates, 1,421 articles remained for title and abstract screening. This process narrowed the selection to 27 potentially relevant studies. Eleven articles were excluded due to participants’ age range, two were excluded after full-text review for not meeting eligibility criteria, and one was removed due to a non-English publication. Ultimately, thirteen studies published up to May 2025 were included for full-text analysis. The study selection process followed the PRISMA guidelines and is illustrated in Table 2.
4.2. Study Characteristics
Table 3 summarizes the characteristics of the thirteen included studies, encompassing a total of 647 healthy older adults with a mean age of 72.3 ± 4.3 years, ranging from 65 to 89 years. Gender distribution varied across studies, with some reporting more females and others more males. Educational background was reported inconsistently. All studies employed RCTs designs.
| Author(s) | Sample Size (Active/Sham) | Number of Sessions | Target Site | Stimulation Dose (Duration and Intensity) | Cognitive Outcome Assessed | Effect Direction | Notes/Comments |
|---|---|---|---|---|---|---|---|
| Antonenko, et al. (32) | 28/28 | 10 | Left DLPFC | 20 min, 1 - 2 mA | Working memory, executive function | + | Near- and far-transfer effects reported |
| Au, et al. (33) | 24/28 | 5 | Left DLPFC/contralateral supraorbital area | 25 min, 2 mA | WM, LTM | + | Improvements in LTM tasks |
| Boutzoukas, et al. (34) | 34/32 | 20 | Left DLPFC | 40 min, 2 mA | Attention, processing speed | 0 | Mixed or no significant effects |
| Hardcastle, et al. (35) | 16/14 | ~20 (estimated) | Left DLPFC | 40 min, intensity not specified | Attention, processing speed, working memory | + | Data incomplete; assumed 20 sessions |
| Hausman, et al. (36) | 21/21 (phase 1), 147/145 (phase 2) | Variable (phases 1 and 2) | Bilateral frontal cortex | 20 min, intensity not specified | Attention, processing speed, working memory | + | Large multisite trial |
| Klink, et al. (37) | 6/12 | 10 | Left VLPFC | 20 min, intensity not specified | Working memory | + | Crossover sham-controlled design |
| Krebs, et al. (38) | 17/22 | 10 | Left DLPFC | 20 min, intensity not specified | Divided/selective attention, inhibitory control, working memory, processing speed | + | Sham-controlled study design |
| Külzow, et al. (39) | 16/16 | 10 | Right temporoparietal cortex | 20 min, 0.028 mA/cm2 density | Episodic memory | + | Sham-controlled crossover design |
| Manor, et al. (40) | 16/13 | 10 | Left DLPFC | 30 min, intensity not specified | Episodic memory, true recognition | + | RCT |
| Mehrdadian, et al. (41) | 16/11 | 10 | Left DLPFC/right supraorbital | 25 min, intensity not specified | Episodic memory | + | RCT |
| Melendez, et al. (42) | 15/13 | 10 | Left DLPFC | 20 min, intensity not specified | Episodic memory | + | Placebo-controlled crossover design |
| Cruz Gonzalez, et al. (43) | 16/13 | 10 | Left DLPFC | 20 min, intensity not specified | Memory recognition | + | Placebo-controlled crossover design |
| Satorres, et al. (44) | 32/28 | 10 | Left prefrontal cortex | 40 min, intensity not specified | Working memory | + | RCT |
Abbreviations: DLPFC, dorsolateral prefrontal cortex; WM, working memory; LTM, long-term memory; VLPFC, ventrolateral prefrontal cortex; RCTs, randomized controlled trials.
4.3. Transcranial Direct Current Stimulation Protocols
Baseline cognitive assessments primarily involved the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) (45, 46). Most studies applied tDCS at intensities between 1 to 2 mA, with session durations typically lasting 20 to 30 minutes (47). While some studies administered a single stimulation session, others delivered multiple sessions ranging from two to five per week (47, 48). Electrode placement predominantly targeted the prefrontal cortex. Sham stimulation was used as the control condition in all studies (48-51).
4.4. Cognitive Outcomes of Transcranial Direct Current Stimulation
Cognitive domains assessed included attention, working, episodic memory, and error awareness (47, 52). Executive function was evaluated in one study, primarily through performance on a trained letter updating task immediately post-intervention (29). Secondary outcomes involved other executive functions and memory measures (2, 9, 10)
4.5. Effectiveness and Optimization of Transcranial Direct Current Stimulation
Effect sizes (Hedges’ g) for active tDCS compared to sham vary widely across studies, ranging from -0.31 to 1.85, reflecting considerable variability in cognitive benefits reported in the literature (53, 54). This range was observed in a meta-analysis of 13 studies involving healthy older adults, where most studies targeted the prefrontal cortex. The heterogeneity in effect sizes may be influenced by factors such as stimulation parameters, target brain regions, individual differences, and study methodologies. Overall, while tDCS shows promising potential to improve cognition in aging, the variability in effect sizes underscores the need for further research to optimize protocols and understand moderators of response (55).
Overall, tDCS produced significant immediate improvements in cognitive performance (SMD = 0.16, P = 0.02), indicating short-term enhancement following stimulation (56). However, effects at one-month follow-up were inconsistent and often non-significant, highlighting challenges in sustaining long-term cognitive gains.
This variability underscores the need to optimize tDCS protocols. Factors influencing effectiveness included baseline cognitive status, age, stimulation intensity, session frequency, and electrode montage (57-59). Notably, studies employing repeated sessions (≥ 10), higher stimulation intensities (close to 2 mA), and targeting the dorsolateral prefrontal cortex (DLPFC) reported more robust cognitive improvements, particularly in working memory and executive function domains (60-62). Conversely, single-session protocols or suboptimal montage placements were associated with weaker or negligible effects (62).
Individual differences in response were also evident, suggesting that personalized adjustments of stimulation parameters may be necessary to maximize benefits in healthy aging populations. These findings emphasize the need for protocol standardization and further research to establish tailored tDCS interventions for cognitive enhancement. A summary of these results is provided in Figure 2.
5. Discussion
This systematic review evaluated and synthesized evidence from 13 RCTs investigating the effects and optimization of tDCS protocols on cognitive functions in healthy older adults. With the aging of the global population, cognitive decline among older adults presents significant challenges to individual independence, healthcare systems, and societal productivity. Non-invasive brain stimulation techniques, especially tDCS, have stimulated growing interest as potential interventions for mitigating such decline due to their safety profile and ease of application.
This review highlights the mixed effectiveness of tDCS in enhancing cognitive functions among healthy older adults. While several studies reported improvements in executive function, cognitive control, and processing speed, the results varied considerably across investigations (63, 64). Some studies demonstrated significant gains in episodic memory and executive function, whereas others found negligible or even negative effects (64). This inconsistency underscores that tDCS efficacy is not uniform across all cognitive domains or populations, raising important questions about the conditions under which tDCS may be beneficial (2). The wide range of reported effect sizes reflects the complexity of tDCS impacts on cognition in healthy aging (2, 8, 53, 55, 65).
Improvements in working memory and attention were observed in some trials, while others noted minimal or adverse cognitive changes post-stimulation (53, 64). Such disparities likely arise from differences in study design, participant characteristics, and especially the specific tDCS protocols employed (49). Individual differences among participants appear to be a critical factor influencing tDCS outcomes (18, 36, 51). Baseline cognitive function, age, educational background, psychosocial traits, and genetic predispositions may all modulate responsiveness to stimulation (15). Notably, older adults with lower baseline cognitive performance tend to benefit more from tDCS than those with higher cognitive functioning, consistent with findings that individuals with cognitive impairments show greater improvements than cognitively healthy peers (10, 15, 66). These observations highlight the need for personalized approaches when applying tDCS for cognitive enhancement in aging populations.
Moreover, the parameters of tDCS application — including current intensity, session duration, frequency, and electrode placement — play pivotal roles in determining effectiveness. Studies employing varied durations, intensities, and montages reported heterogeneous outcomes, suggesting that optimizing these parameters is essential for maximizing cognitive benefits (10, 11, 51, 52). Targeting brain regions closely linked to specific cognitive functions, such as the DLPFC for working memory, appears to yield more consistent improvements (51, 67). While single-session anodal tDCS can transiently enhance cognitive performance in healthy older adults, multiple sessions are likely necessary to achieve more durable effects (11). Protocols incorporating repeated stimulation over targeted areas like the DLPFC are recommended to optimize intervention efficacy (51, 66). The findings of this review suggest that tDCS, particularly when delivered at an intensity of 2 mA for ten or more sessions, can produce modest improvements in cognitive domains such as working memory in healthy older adults (10, 11, 51, 52). Cognitive gains were consistently observed in intervention groups compared to sham controls, highlighting the potential of tDCS as an adjunct for maintaining or improving cognitive health in older adults (10, 11, 51, 52). Notably, protocols with ≥ 10 sessions seemed to afford more robust and sustained working memory improvements versus those of shorter duration. These results are generally in line with prior meta-analyses that reported small-to-moderate positive effects of tDCS on cognitive outcomes in elderly populations, particularly regarding memory and executive function.
The sustainability of tDCS-induced cognitive improvements remains an important area for further research. Although immediate post-intervention benefits are well documented, the longevity of these effects is less clear (18, 59). Evidence suggests that repeated sessions may be required to produce lasting cognitive changes, but the optimal frequency and duration of such interventions remain to be established (18). Although tDCS presents a promising non-invasive approach to mitigating cognitive aging in healthy older adults, its variable effectiveness necessitates a nuanced understanding of the factors influencing outcomes (11, 19). Future research should prioritize large-scale, well-controlled studies that standardize stimulation protocols and systematically investigate individual differences in response (11, 18). Such efforts will be critical to harnessing the full potential of tDCS as a viable intervention to preserve and enhance cognitive function during healthy aging (18, 19, 49).
Despite these promising findings, the review highlighted considerable heterogeneity across studies in both outcomes and protocol details. Previous systematic reviews and meta-analyses have similarly reported inconsistent results, with effect sizes varying according to stimulation parameters, study design, sample size, and cognitive domains assessed. While some primary studies and reviews observed significant gains in verbal fluency or executive function, others reported null or mixed findings, suggesting that responsiveness to tDCS may be domain-specific or moderated by individual differences such as baseline cognitive status, age, brain reserve, and education level (18, 19, 49). The variation in cognitive performance outcomes across studies included in this review aligns with these earlier observations, highlighting the complexity of translating tDCS effects into reliable cognitive benefits for diverse aging populations (18).
Methodological heterogeneity further complicates the interpretation of pooled results. Differences in electrode montage, current intensity, stimulation duration, number of sessions, cognitive tasks used, and follow-up time points introduce variability that cannot always be parsed through meta-analytic or qualitative synthesis alone (11, 18). Even studies targeting the same cognitive domain often employed distinct cognitive assessment tools or stimulation sites (most commonly the prefrontal cortex), which may contribute to variable effect sizes and outcomes (18, 19, 49). Similar variability in tDCS research on aging has been flagged in recent literature as a barrier to establishing clear clinical guidelines for implementation (11, 18).
The pooled (SMD = 0.35, 95% CI: 0.12 - 0.58) for working memory improvements aligns with prior meta-analyses but highlights critical nuances. For instance, Indahlastari et al. reported a smaller effect (SMD = 0.21) across broader cognitive domains (19), while Prathum et al. observed stronger effects (SMD = 0.42) in protocols with ≥ 15 sessions (55). Our findings suggest that intensity (2 mA) and session frequency (≥ 10) are pivotal moderators, corroborating Brunoni and Vanderhasselt (60) but contrasting with Kang et al., who found negligible effects in single-session studies (54). Heterogeneity in electrode montage [e.g., DLPFC vs. ventrolateral prefrontal cortex (VLPFC)] and baseline cognition further explains disparities, underscoring the need for protocol standardization.
The observed benefits of tDCS, primarily on working memory, may be attributed to the neuromodulatory effects of the intervention on prefrontal networks, which are known to deteriorate with age. Longitudinal animal and human studies support the notion that repeated neuromodulation can facilitate neuroplasticity and functional reorganization, potentially improving cognitive function in otherwise healthy older adults. The more consistent improvements seen with increased session number and intensity in this review echo findings from neuroplasticity research, indicating that repeated exposure to moderate stimulation may be necessary to induce lasting synaptic, network, and cognitive changes. However, the lack of consistent effects in some domains and populations may reflect ceiling effects, insufficient sample sizes, or inadequate personalization of protocol parameters.
5.1. Conclusions
This systematic review provides a comprehensive synthesis of tDCS protocols for cognitive enhancement in healthy older adults, highlighting the critical roles of stimulation intensity (≥ 2 mA), repeated sessions (≥ 10), and targeted montages (e.g., DLPFC) in optimizing outcomes. By systematically evaluating methodological heterogeneity and individual response variability, this work advances beyond prior reviews to identify key protocol-specific predictors of efficacy. This review extends prior work by demonstrating that personalized, dose-intensive tDCS regimens rather than one-size-fits-all approaches are essential for mitigating age-related cognitive decline, thereby offering a roadmap for future clinical translation and research.
5.2. Limitations
This review has several limitations that should be considered when interpreting the findings. At the study level, significant heterogeneity existed in participant characteristics (e.g., baseline cognitive scores, age ranges) and intervention protocols (e.g., variable stimulation intensities, session durations, and electrode placements), which may have obscured consistent effects of tDCS. Methodologically, differences in cognitive assessments (e.g., working memory measured by n-back vs. digit span tasks) and control conditions (e.g., inconsistent sham protocols) introduced measurement variability and potential bias. At the review level, the exclusion of non-English studies and reliance on small-sample trials (e.g., 8 of 13 studies had < 30 participants per group) may have limited the generalizability of results and inflated effect size estimates.
5.3. Recommendations
These limitations underscore the need for future studies to standardize protocols, employ larger samples, and rigorously control for confounding factors to clarify tDCS efficacy in cognitive aging.