1. Background
Urinary tract infections (UTIs) are the most common and severe public health problem. They are caused by bacterial infections and affect around 150 million individuals worldwide every year. Urinary tract infections are considered a significant cause of morbidity in males and females of all ages, even in infants. These infections are caused by both gram-positive bacteria and gram-negative and by certain fungi. The most common causative agent for both uncomplicated and complicated UTIs is uropathogenic Escherichia coli (UPEC). Other bacteria causing UTIs are Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis, and Staphylococcus saprophyticus.
Metal oxide nanomaterials of CuO, Fe3O4, TiO2, and ZnO have been scrutinized for their biological activities. Among all the metal oxides, ZnO is widely used in the elimination of heavy metals (toxic elements), such as sulfur and arsenic, from the polluted water owing to their large surface area by the volume ratio than the bulk materials (1-5). Biosynthesis or green of nanoparticles (NPs) is an alternative novel approach to the synthesis of NPs using plant extracts, algae, fungi, yeast, and bacteria (6, 7). Specifically, the plant extracts mediated-NP preparation approach is a more effective, biocompatible, safe, and low-cost method than other chemical and physical methods (8). The literature evidence shows that plant extracts were successfully used as reducing and stabilizing agents in the synthesis of NPs, such as nickel, iron, copper, gold, silver, cobalt, lead, titanium, palladium, magnetite, platinum, and zinc (9, 10). Plant extract-mediated nanomaterials revealed a potential for pharmacological effects on microbes, cancer, malaria, wound healing, hepatitis, inflammation, and other acute diseases. Zinc oxide nanoparticles (ZnONPs) also have the potential application in the pharmacological (medicine) field, including drug delivery, diagnosis of diseases, and biological effects, such as antioxidant and antimicrobial, which can also be applied in UTI treatments. The high ionic nanosized metal oxides, such as ZnONPs, were unique because they were synthesized with an unusual crystal structure, along with the large surface areas. Moreover, the mineral element zinc is very essential to human health, and the oxide form of zinc, such as ZnO, is the daily supplement for zinc.
Zinc oxide nanoparticles exhibit good biocompatibility to the human cells. In addition, such NPs reveal cell bactericidal effects and internalization effects against the numerous microbial strains that are involved in UTIs. Due to these advantages, several researchers were interested in using zinc-based NPs for UTI therapy.
Echinochloa esculenta (Japanese Barnyard millet) and Echinochloa frumentacea (Indian Barnyard millet) are the two main species of Barnyard millet belonging to the family Poaceae (11-15). Tamil Nadu, Bihar, Punjab, Gujarat, Orissa, Maharashtra, and Madhya Pradesh are the main cultivators of Barnyard millet in India. Barnyard millet has micronutrients, such as iron and zinc, and contains an enormous amount of macronutrients, alkaloids, steroids, glycosides, and tannins, which contribute to the different medicinal properties, such as anti-carcinogenic, anti-inflammatory, antioxidant, antimicrobial, and wound healing capacity (16, 17). The present study aimed to biosynthesize and characterize the Echinochloa esculenta-mediated ZnONPs and their antibacterial potential against UTI-causing microbes.
2. Methods
2.1. Preparation of Precursor and Echinochloa esculenta Extract
Zinc acetate dihydrate (Zn (C2H3O2)2.2H2O) and ethanol (C2H5OH) were of analytical grades purchased from Merck. The fresh Echinochloa esculenta millets were purchased from the local herbal store Aarogya Herbals, Trichy, Tamilnadu, India. Collected materials were washed properly and shade-dried. Moreover, 500 g of dried Echinochloa esculenta millets were taken in a 500 ml beaker, and 70% ethanol solvent was added, mixed well, and kept aside for at least 3 to 5 days. After the extraction process was completed, the extract was filtered, and the filtrate was stored in a refrigerator for future study (18-20).
2.2. Synthesis of Echinochloa esculenta-Mediated ZnONPs
In this study, 50 mL of 0.1 M zinc acetate solution was taken in a 250 ml beaker and placed on a magnetic stirrer at 60°C. Additionally, 10 ml of Echinochloa esculenta extract was slowly added at every 30-minute interval. After the reaction time was over, the final reaction mixture (colloidal liquid) was stood at room temperature for 12 hours. Zinc oxide nanoparticles were obtained by centrifuging at 6 000 rpm for 15 minutes. The precipitate was dried in an oven for the complete conversion of zinc hydroxide (Zn (OH)2) into ZnO. Finally, the purified ZnONPs were further taken for characterization and microbial activity.
2.3. Characterization of Echinochloa esculenta-Mediated ZnONPs
PerkinElmer makes an ultraviolet-visible (UV-visible) spectrometer in the model of lambda 35 with a resolution and wavelength of 1 cm and 200-800 nm, respectively. The dried sample's Fourier transform-infrared resonance spectroscopy (FTIR) spectrum was documented in the range between 450 and 4000 cm−1 with a resolution of 4 cm−1. The crystallographic structure of a material was determined by X-ray diffraction (XRD) (Shimadzu), and the morphology of the ZnONPs was examined by scanning electron microscopy (SEM) (JEOL JSM- 6480 LV SEM). The elemental analysis of bio-synthesized ZnONPs was analyzed by EDX (Bruker) (21-24).
To examine the antibacterial activity of Echinochloa esculenta-mediated ZnONPs, five strains (Escherichia coli [MTCC 25922], Enterobacter aerogenes [MTCC 29212]. Pseudomonas aeruginosa [MTCC 27853], Staphylococcus aureus [MTCC 25923], and Proteus vulgaris (MTCC 7299]) were prepared as test organisms. The strains were procured from the microbial type culture and collection (MTCC) at Chandigarh, India. Bacterial strains were cultivated at 37ºC and maintained on nutrient agar (Difco, USA) slant at 4ºC. The antibacterial potential of synthesized ZnONPs was investigated against the UTI-causing above-mentioned bacteria by the disc diffusion method (25-28). The anti-bacterial data of the zone of inhibition of extract, zinc acetate, standard, and ZnONPs were analyzed using MS Excel 2010 and presented as mean ± standard deviation (SD) of three diameters of each zone.
3. Results and Discussion
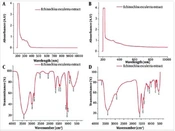
The color changes after adding the plant extract in the reaction mixture preliminarily confirmed the formation of ZnONPs. The color change from yellow to white indicated the formation of ZnONPs. The UV spectra of Echinochloa esculenta extracts and qualitative analysis revealed that flavonoid aromatics (nucleus) are mostly present in it, which is shifted to 324 nm in the UV spectrum of synthesized ZnONPs. Flavonoid biomolecules in the extract played a role in the conversion of Zn2+ to Zno (Figure 1) (29-31).
The FTIR spectrum of Echinochloa esculenta extracts and synthesized ZnONPs were shown in Figure 1, and in-plant extract vibrational peaks were observed at 3399, 2948, 1651, 1049, 1032, 1021, 879, and 678 cm-1 which denoted the existence of O-H stretching of alcohol, C-H stretching of alkane, C=C stretching of alkene, C-O stretching of alcohol, CO-O-CO stretching of anhydride, C-N stretching of amine, C=C bending of alkene, and C-X stretching of halo compound, respectively. Similarly, in the FTIR spectrum of ZnONPs, the peaks were observed at 3431, 2925,1592,1405,1341, 1119, 1049,679, and 497 cm-1 which was associated at O-H Stretching of alcohol, C-H Stretching of alkane, N-H bending of amine, O-H bending of alcohol, O-H bending of phenol, C-O stretching of aliphatic ether or secondary alcohol, CO-O-CO stretching of anhydride, C=C bending of alkene, and M-O Stretching of Zn-O. The Echinochloa esculenta extract showed a broad peak at 3431 cm-1, which indicates the presence of O-H groups, and after the synthesis of ZnONPs, there is a shift in the broad peak to the 3399 cm-1, which indicates that the flavonoids or phenolic compounds O-H group involved the reducing process. The peak at 1119 cm-1 is shifted to 1019 cm-1 in synthesized ZnONPs, indicating that the C=O functional group might get involved in the reduction process (32).
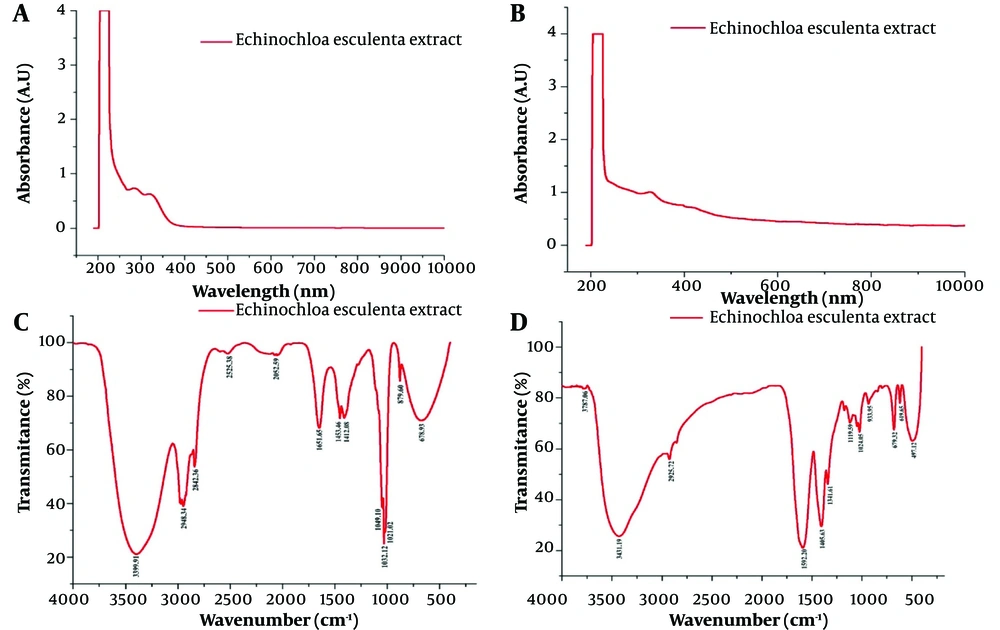
The resulting XRD pattern of Echinochloa esculenta-mediated ZnONPs showed main peaks corresponding to 2θ values of 31.86o, 34.48o, 36.35o, 47.61o, 56.68o, 62.92o, and 69.09o as shown in Figure 2. The obtained XRD patterns of Echinochloa esculenta-mediated ZnONPs are well correlated with the standard patterns of ZnO, which means that all the peaks match the standard JCPDS card No. 36-145. Furthermore, it confirmed that formed ZnONPs are in hexagonal wurtzite pattern/structure. The average crystallite size of the observed ZnONPs calculated using the Debye Scherrer formula was about 23.38 nm (33).
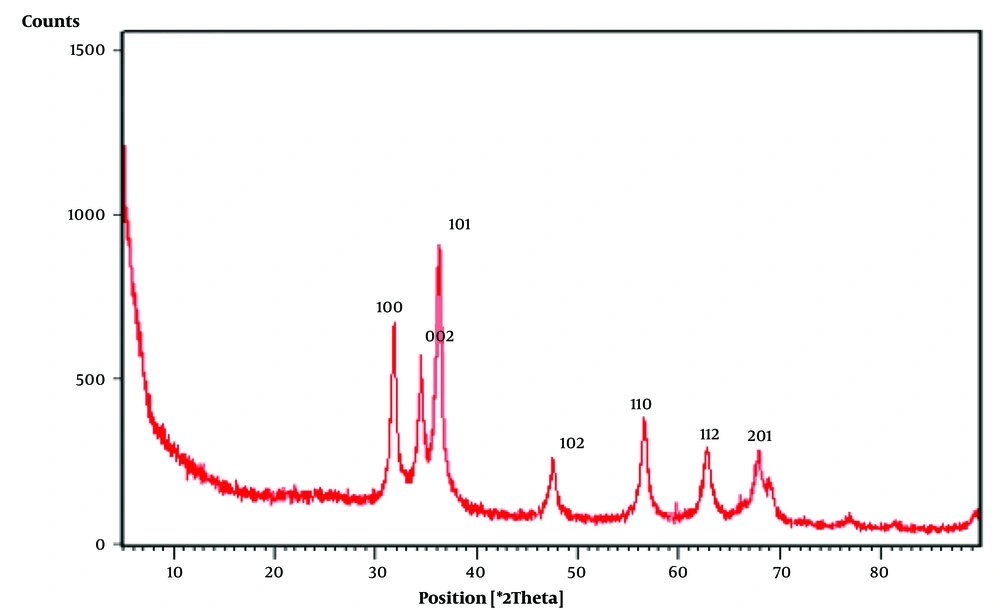
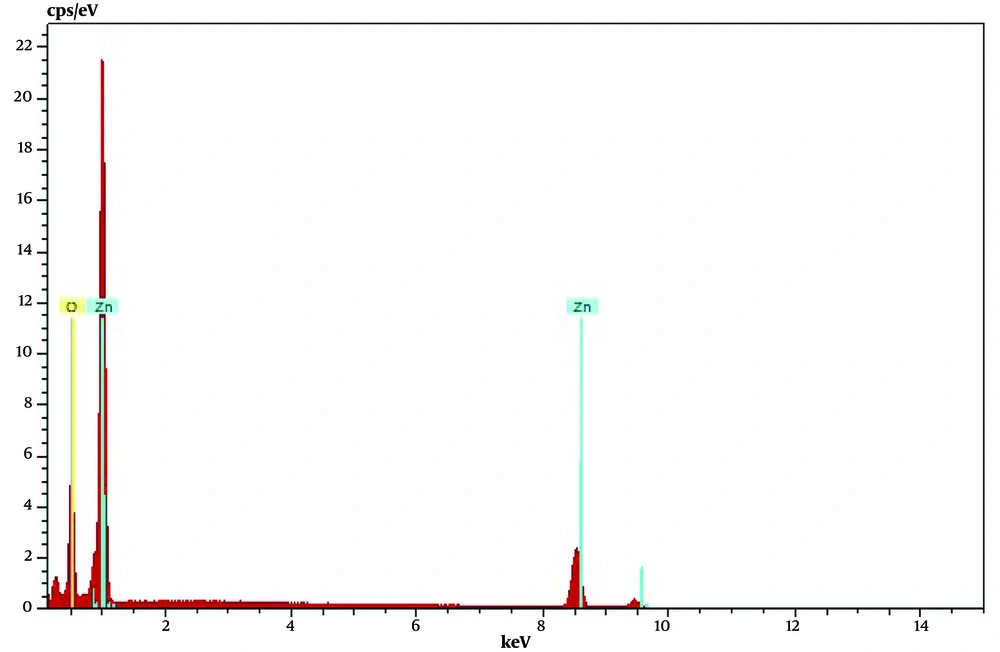
The SEM image of ZnONPs synthesized by Echinochloa esculenta showed relatively polydispersed shape (mostly spherical) NPs (Figure 3). The shapes and sizes of NPs play a crucial role in the effectiveness of microbial activity against any microbes/pathogens. Because spherical nanoparticles tend to be very potent during antibacterial activity owing to their ability to easily penetrate into the cell wall of pathogens, ZnONPs synthesized from the Echinochloa esculenta millet can be of great importance in treating clinical pathogens. Energy dispersive X-ray spectroscopy (EDX) was used to find the elemental composition in the formed ZnONPs. The EDX of ZnONPs revealed the presence of pure zinc at 73.23% and oxygen at 26.77%, as shown in Figure 4, which evidenced that formed nanoparticles were obtained with purity (less impurity).
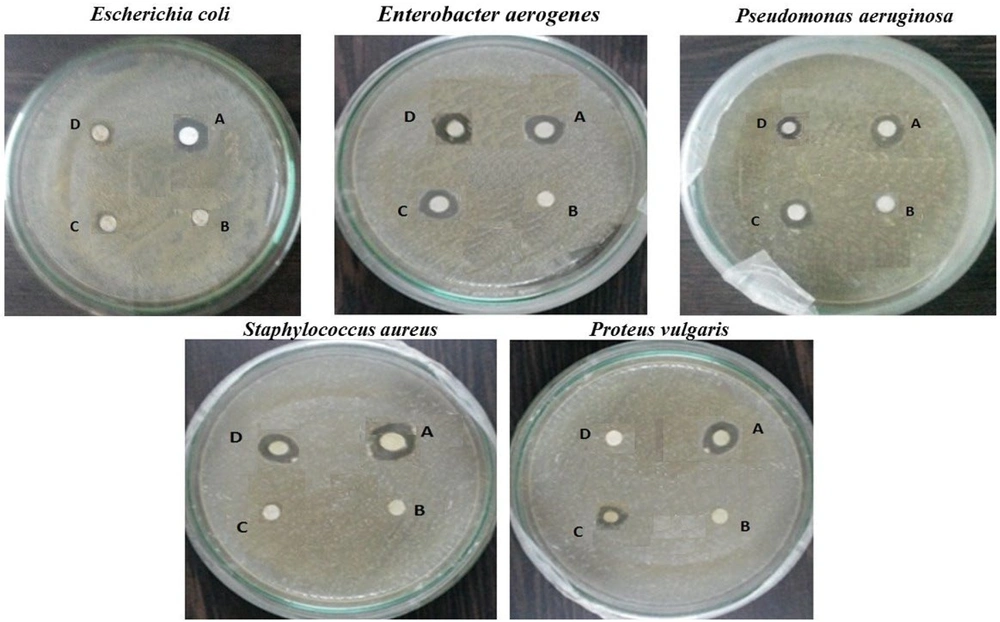
The antibacterial activity of synthesized ZnONPs was investigated against UTI microbes, such as E. coli, E. aerogenes, P. aeruginosa, S. aureus, and P. vulgaris, with respect to amoxicillin. Synthesized ZnONPs were tested against the five UTI-causing pathogens, and their results revealed a noticeable zone of inhibition against the pathogens compared to zinc acetate (precursor) and the Echinochloa esculenta extract. The maximum inhibition effect of ZnONPs was observed against the pathogen S. aureus (gram-positive); however, no zero inhibitory effect was observed against P. vulgaris (gram-negative), which is shown in Table 1. Similarly, Souza et al. (34), Azizi-Lalabadi et al. (35), Demissie et al. (36), and Farzana R et al. (37) synthesized the ZnONPs by using plant extracts and reported its anti-microbial activities against the gram-positive and negative pathogens.
| Microorganisms | Zone of Inhibition (mm) (10 µL) | |||
|---|---|---|---|---|
| A (Amoxicillin) | B (Zinc Acetate) | C (Plant Extract) | D (ZnONPs) | |
| Escherichia coli | 8.0 ± 0.33 | 0 | 1.0 ± 0.13 | 2.0 ± 0.16 |
| Enterobacter aerogenes | 8.0 ± 0.62 | 0 | 3.0 ± 0.20 | 4.0 ± 0.20 |
| Pseudomonas aeruginosa | 7.0 ± 0.23 | 0 | 4.0 ± 0.15 | 3.0 ± 0.13 |
| Staphylococcus aureus | 8.0 ± 0.30 | 0 | 0 | 5.0 ± 0.17 |
| Proteus vulgaris | 8.0 ± 0.66 | 0 | 2.0 ± 0.26 | 0 |
Antibacterial Activity of Echinochloa esculenta Extract, Zinc Acetate, Zinc Oxide Nanoparticles, and Standard Amoxicillin on Urinary Tract Infection Pathogens
According to the results of the above-mentioned studies, it is observed that zinc oxide nanomaterial reported noticeable inhibitory actions with the zone of inhibition of 12 mm to 25 mm. A different mechanism was observed in the action of NPs against the bacteria, and their reports state that the differences in the structural/composition of NPs can affect their inhibitory potential. The zone of inhibition of Echinochloa esculenta-mediated ZnONPs was much lower than that of amoxicillin (standard antibiotics), which indicates the need for further modification of NPs to obtain the desired biological effects (Figure 5). The Echinochloa esculenta-mediated ZnONPs inhibit the microbial growth of five bacteria in the in-vitro antimicrobial assay (38, 39).
3.1. Conclusions
The synthesis of ZnONPs from Echinochloa esculenta through an eco-friendly approach was observed to be successful, and the UV-vis peak at 324 nm confirmed the synthesized particles were ZnONPs. The FTIR spectral data confirmed the presence of functionalities of phytochemicals, such as flavonoids and phenolics, which played a role in the reduction of zinc. The antibacterial activity of ZnONPs against urinary infection pathogens has proved that it can be used as a potent antibacterial agent. Therefore, this study had the potential value of developing new eco-friendly, low-cost, and less toxic nanomaterials that can be used as a bio-control for diseases.