1. Background
Bone marrow aspiration and biopsy remain cornerstone procedures in pediatric hematology and oncology, serving as essential tools for the diagnosis and management of both malignant and non-malignant hematologic conditions. Yet, their invasive nature and associated physical and psychological discomfort, especially in younger patients, present a formidable challenge — demanding sedation strategies that are both effective and well-tolerated (1, 2). In this context, procedural sedation serves a dual role: Not only does it improve the immediate procedural experience by reducing pain and anxiety, but it is also crucial to minimize psychological distress and delirium, ensure cooperation, and prevent adverse procedural outcomes (3).
A wide spectrum of pharmacologic agents has been leveraged to achieve optimal sedation and analgesia in children. Midazolam, owing to its strong anxiolytic, amnestic, and sedative properties, is a mainstay in pediatric sedation protocols and is frequently paired with other medications to enhance efficacy and safety (4). Ketamine, distinct for its dissociative anesthesia, robust analgesic effect, and preservation of respiratory and cardiovascular stability, occupies a central role in pediatric practice (5). Its combination with midazolam is routine, aiming to mitigate emergence phenomena, whereas adjunctive atropine is often utilized to counteract hypersalivation and minimize airway complications (6).
While these protocols have attained widespread acceptance, accumulating contemporary evidence has broadened our understanding of sedative regimens in invasive procedures. Notably, recent randomized clinical trials have compared the efficacy of ketamine and dexmedetomidine, alone and in combination with other agents, in diverse procedural contexts. Shafiee et al. highlighted the favorable safety and efficacy profile of ketamine compared to other sedatives in adult endoscopic procedures (7). In a parallel trial, Aminnejad et al. demonstrated the comparable efficacy and safety of dexmedetomidine-ketamine (DEXKET) versus propofol/fentanyl for sedation, supporting the adaptability of these regimens beyond traditional boundaries (8). Furthermore, the unique pharmacologic profile of dexmedetomidine — marked by minimal respiratory depression and hemodynamic stability — has recently attracted attention for its therapeutic and sedative utility across various clinical settings, including critically ill patients (9).
Despite the rising prominence of dexmedetomidine, ketamine continues to be an irreplaceable component of pediatric sedation, particularly valued for its rapid onset and predictability — qualities of pronounced relevance in resource-limited settings or urgent interventions (6, 7, 10). Nevertheless, ongoing debates surround the comparative safety profiles of sedative combinations, especially with respect to cardiorespiratory risks and the potential for unpredictable sedation depth or paradoxical reactions (8, 9, 11).
Given these concerns and the expanding landscape of sedation pharmacology, it is increasingly clear that comparative, rigorously designed studies are essential to delineate optimal strategies for children undergoing painful procedures such as bone marrow aspiration. Yet, published research still falls short in providing head-to-head comparisons of widely used regimens — specifically, ketamine-atropine-midazolam (KAM) versus dexmedetomidine-atropine-midazolam (DAM) — regarding effectiveness, patient and parent satisfaction, and adverse event rates (1, 2, 7). Through robust clinical inquiry and standardized outcome assessment, such work is poised to define best practices and elevate quality of care in the pediatric setting.
2. Objectives
As such, the present study is conducted to address this critical gap by directly comparing the sedative efficacy of KAM and DAM in children undergoing bone marrow biopsy (BMB), with particular focus on comprehensive sedation outcomes. This research endeavors to contribute meaningful evidence to the field and inform future guidelines in pediatric procedural sedation.
3. Methods
This parallel, double‑blind, randomized clinical trial was conducted at Khorami Hospital, Qom, Iran, from November 22, 2023, to December 21, 2024. The study protocol was reviewed and approved by the Institutional Review Board of Qom University of Medical Sciences (IR.MUQ.REC.1402.170). The trial was retrospectively registered in the Iranian Registry of Clinical Trials (IRCT20250719066544N1) during data collection, following ethical approval and in compliance with national regulatory procedures. Eligible participants were randomly assigned to two groups with an equal allocation ratio (1:1), and both participants and outcome assessors remained blinded to group identity throughout the study period.
3.1. Study Population and Randomization
Participants were recruited via census sampling from eligible children referred for BMB. Sample size was determined using a two‑sided t‑test formula with α = 0.05, statistical power of 0.80, and mean ± standard deviation (SD) sedation scores of 3.34 ± 0.76 and 3.80 ± 0.63 based on prior similar studies, yielding 36 patients per group (total = 72). Seventy‑two pediatric patients were enrolled and randomly allocated into two equal groups (n = 36 each) using computer‑generated block randomization to ensure balanced group assignment. Allocation concealment was maintained using sealed, coded envelopes prepared by the principal investigator.
Inclusion criteria comprised children aged 1 - 8 years who were candidates for bone marrow biopsy and whose parents provided written informed consent. Exclusion criteria included congenital cardiac or respiratory disorders, the presence of fever or productive cough, abnormal lung auscultation findings (wheezes or crackles), and lack of parental consent.
The anesthesiologist was informed only of the group codes (A/B) and remained blinded to the actual study medications. The pediatric oncologist performing the biopsy and all patients were completely unaware of group assignments. The principal investigator retained the master code list, with emergency unblinding permitted solely in case of a serious adverse reaction.
The KAM group received oral ketamine (4 mg/kg) plus atropine (0.1 mg/kg) and midazolam (0.5 mg/kg), while DAM group received oral dexmedetomidine (8 μg/kg) with identical doses of atropine (0.1 mg/kg) and midazolam (0.5 mg/kg). All study medications were administered 45 minutes prior to the procedure to ensure peak sedative effects during biopsy. The BMB procedure began when the sedative drugs had taken effect and the children reached an appropriate level of sedation, defined as a Richmond Agitation-Sedation Scale (RASS) score of -1 or -2 (light sedation) and a Ramsay score of 4 (brisk response to stimulus). All procedures in both groups were performed by the same physician to maintain consistency.
3.2. Data Collection and Monitoring
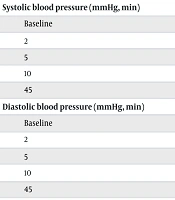
Demographic characteristics, including age, sex, weight, height, and Body Mass Index (BMI), were documented at baseline. Clinical parameters comparing heart rate, respiratory rate, systolic and diastolic blood pressure, temperature, and peripheral oxygen saturation were recorded at baseline (0 min) and at 2, 5, 10, and 45 minutes after drug administration.
Standardized protocol included supplemental oxygen administration via nasal cannula immediately following drug administration. Continuous cardiorespiratory monitoring was maintained throughout the procedure, with emergency resuscitation equipment immediately available. All BMB procedures were performed by a single pediatric hematology specialist to ensure technical consistency across study participants.
3.3. Sedation Assessment
Sedation depth was assessed using two validated clinical instruments: The Ramsay Sedation Scale (RSS) and the RASS.
The Ramsay score, a 6-point ordinal scale, quantifies sedation levels from anxious/agitated (score 1) to deep unresponsiveness (score 6). Per protocol, a score ≥ 4 was defined as indicating adequate sedation for procedural safety and efficacy (12, 13).
The RASS provides a 10‑point continuum [+4 (combative) to -5 (unarousable)] specifically designed to evaluate both agitation and sedation states (14).
For statistical analysis in SPSS, negative numeric values could not be properly configured; therefore, RASS scores were recoded into a 1 - 10 numeric format corresponding to the original -5 to +4 categories, preserving the ordinal relationship between levels of agitation and sedation.
Trained assessors, blinded to treatment allocation, documented sedation scores at the same five standardized time points as physiological monitoring. This dual-scale approach enhanced measurement reliability and strengthened the validity of sedation assessments.
The detailed scoring criteria for each scale are presented in Appendix 1 (RSS) and Appendix 2 (RASS), found in Supplementary File.
3.4. Physician Satisfaction
To assess the clinical acceptability of the sedation protocols, procedural satisfaction was evaluated by the attending pediatric oncologist immediately post‑procedure. Two complementary measures were applied: (1) A 5‑point Likert scale (1 = “Very dissatisfied” to 5 = “Very satisfied”) and (2) a 10‑point numerical rating scale (1 = least satisfied and 10 = most satisfied). The numerical ratings (1 - 10) were used for quantitative group comparisons in the analysis.
3.5. Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics (version 26; Armonk, NY). Continuous variables were expressed as mean ± SD, while categorical variables were presented as counts and percentages. Data normality was evaluated using the Shapiro-Wilk test. Between-group comparisons of continuous variables employed either independent samples t-tests (parametric data) or Mann-Whitney U tests (non-parametric data), as appropriate. Categorical variables were analyzed using either Pearson's chi-square test or Fisher's exact test, depending on expected cell frequencies. Statistical significance was defined as a two-tailed P-value < 0.05 for all analyses.
4. Results
A total of 72 pediatric patients were randomized equally into two groups: The KAM and DAM. The KAM group included 19 boys (52.8%) and 17 girls (47.2%), whereas the DAM group included 17 boys (47.2%) and 19 girls (52.8%), showing no significant difference in gender distribution (P = 0.81). The mean age was 4.61 ± 2.10 years in the KAM group and 4.81 ± 2.08 years in the DAM group (P = 0.69). The mean BMI was slightly higher in the DAM group (15.60 ± 1.01 kg/m2) than in the KAM group (15.24 ± 1.87 kg/m2), but this difference was not statistically significant (P = 0.32). Other baseline variables were comparable between groups (Table 1).
| Variables | KAM Group | DAM Group | P-Value |
|---|---|---|---|
| Gender (male), No. (%) | 19 (26.4) | 17 (23.6) | 0.81 |
| Age (y) | 4.61 ± 2.10 | 4.80 ± 2.08 | 0.69 |
| BMI (kg/m2) | 15.24 ± 1.87 | 15.59 ± 1.01 | 0.32 |
Abbreviations: KAM, ketamine-atropine-midazolam; DAM, dexmedetomidine-atropine-midazolam; BMI, Body Mass Index.
a Values are expressed as mean ± standard deviation (SD) unless indicated.
Vital signs were compared between the KAM and DAM groups at baseline (0 min) and at 2, 5, 10, and 45 minutes after drug administration (Table 2). Except for pulse rate, no statistically significant difference was detected between the groups at any of the assessed time points. Pulse rate was significantly higher in the DAM group at 2, 5, 10, and 45 minutes (P < 0.05 for all comparisons), whereas baseline values were comparable (P = 0.76). Despite these transient increases in the DAM group, all mean heart rates remained within normal physiological limits, without clinical signs of hemodynamic instability. No significant changes were observed in systolic or diastolic blood pressure, respiratory rate, body temperature, or peripheral oxygen saturation (P > 0.05 for all). Both regimens maintained stable cardiorespiratory and thermoregulatory parameters throughout the monitoring period, indicating comparable overall hemodynamic safety profiles.
| Vital Signs and Time Points | KAM | DAM | P-Value |
|---|---|---|---|
| Pulse rate (/min) | |||
| Baseline | 122.9 ± 18.8 | 124.1 ± 17.0 | 0.76 |
| 2 | 122.3 ± 14.0 | 129.6 ± 14.1 | 0.31 |
| 5 | 124.2 ± 11.9 | 132.7 ± 14.8 | 0.00 |
| 10 | 124.0 ± 13.4 | 133.5 ± 13.0 | 0.00 |
| 45 | 116.3 ± 12.2 | 124.8 ± 13.3 | 0.00 |
| Systolic blood pressure (mmHg, min) | |||
| Baseline | 94.0 ± 10.9 | 93.6 ± 7.4 | 0.89 |
| 2 | 94.6 ± 11.8 | 94.0 ± 8.8 | 0.81 |
| 5 | 95.4 ± 14.1 | 94.4 ± 9.3 | 0.71 |
| 10 | 94.8 ± 13.4 | 94.4 ± 9.6 | 0.88 |
| 45 | 90.4 ± 12.0 | 88.5 ± 9.0 | 0.44 |
| Diastolic blood pressure (mmHg, min) | |||
| Baseline | 53.3 ± 15.0 | 52.8 ± 13.6 | 0.87 |
| 2 | 54.0 ± 15.6 | 51.7 ± 14.3 | 0.51 |
| 5 | 55.3 ± 16.7 | 50.8 ± 15.5 | 0.23 |
| 10 | 55.2 ± 16.5 | 52.3 ± 16.5 | 0.45 |
| 45 | 53.6 ± 17.1 | 48.0 ± 15.7 | 0.15 |
| Respiratory rate (/min) | |||
| Baseline | 28.4 ± 6.1 | 29.1 ± 5.6 | 0.62 |
| 2 | 29.5 ± 6.2 | 29.6 ± 5.5 | 0.92 |
| 5 | 29.3 ± 6.8 | 29.3 ± 6.0 | 0.95 |
| 10 | 28.9 ± 6.2 | 28.9 ± 5.5 | 1.00 |
| 45 | 28.0 ± 4.3 | 28.0 ± 5.3 | 0.96 |
| Temperature (ᵒC, min) | |||
| Baseline | 36.8 ± 0.5 | 36.8 ± 0.4 | 0.96 |
| 2 | 36.8 ± 0.5 | 36.8 ± 0.4 | 0.77 |
| 5 | 36.8 ± 0.5 | 36.7 ± 0.5 | 0.75 |
| 10 | 36.8 ± 0.5 | 36.8 ± 0.5 | 0.60 |
| 45 | 36.8 ± 0.4 | 36.9 ± 0.5 | 0.52 |
| SpO2 (%, min) | |||
| Baseline | 98.3 ± 1.4 | 98.2 ± 1.6 | 0.41 |
| 2 | 98.1 ± 1.4 | 98.0 ± 1.4 | 0.74 |
| 5 | 98.2 ± 1.4 | 97.6 ± 1.5 | 0.11 |
| 10 | 97.8 ± 1.1 | 97.6 ± 1.1 | 0.40 |
| 45 | 98.0 ± 1.1 | 97.7 ± 1.3 | 0.41 |
Abbreviations: KAM, ketamine-atropine-midazolam; DAM, dexmedetomidine-atropine-midazolam.
a Values are expressed as mean ± standard deviation (SD).
4.1. Sedative Efficacy Outcomes
This randomized trial evaluated two critical pharmacodynamic parameters between the KAM and DAM groups: Time to achieve effective sedation onset and total duration of maintained sedation.
The mean ± SD time required to achieve adequate sedation for bone marrow aspiration was significantly shorter in the KAM group compared to the DAM group (18.88 ± 5.22 minutes vs. 39.38 ± 11.08 minutes, respectively; P < 0.001). This demonstrates that the KAM protocol produced more rapid sedation onset — a clinically important advantage in urgent procedures or high-volume settings. The KAM group also showed statistically longer sedation duration than the DAM group (109.02 minutes vs. 100.41 minutes, respectively; P = 0.02). This extended duration may provide better coverage during lengthy procedures, potentially reducing the need for supplemental doses. Both the faster onset and prolonged duration of sedation with KAM were statistically superior to DAM (P < 0.001 for both comparisons).
4.2. Practitioner Satisfaction and Subjective Assessment
Clinicians reported significantly greater satisfaction with the KAM sedation protocol across all evaluation methods. Quantitative ratings based on the 10‑point numerical scale averaged 7.6 ± 1.2 for KAM compared with 6.1 ± 1.2 for DAM (mean difference = 1.5, 95% CI: 1.0 - 2.0; P < 0.001). Qualitative assessments using the 5‑point Likert scale — covering factors such as ease of administration, patient cooperation, and workflow integration — further confirmed this preference, showing mean scores of 4.1 ± 1.0 for KAM versus 3.3 ± 0.8 for DAM (P < 0.001). These consistent findings indicate that KAM offers broader advantages beyond pharmacological effects, enhancing both practitioner experience and procedural efficiency.
4.3. Comparative Results
Children receiving KAM achieved adequate sedation significantly faster than those receiving DAM. The mean time to reach target sedation was 18.9 ± 5.2 minutes for KAM compared to 39.4 ± 11.1 minutes for DAM — a difference of 20.5 minutes (95% CI: 16.7 to 24.3 minutes; P < 0.001). This represents a large effect size (Cohen's d = 2.3). This clinically important reduction in sedation time offers key benefits as procedures can begin more quickly, less anxiety for both children and parents before procedures, especially valuable in emergency departments, high-volume clinical settings, and situations where faster sedation is critical. The highly significant result (P < 0.001) confirms KAM's reliably faster onset compared to DAM.
4.4. Depth and Quality of Sedation
When evaluating sedation depth, KAM demonstrated significantly greater effectiveness as measured by two validated scales. The average Ramsay score was 5.0 ± 0.8 for KAM compared to 3.1 ± 0.8 for DAM (mean difference: 1.9 points, 95% CI: 1.5 to 2.3; P < 0.001). Similarly, the mean RASS scores were higher with KAM (8.4 ± 1.1) versus DAM (5.8 ± 1.3; mean difference: 2.6, 95% CI: 2.0 to 3.2; P < 0.001). These findings clearly show that KAM produces deeper, more controlled sedation and potentially lowers risks of patient movement, distress, or memory recall — especially crucial factors when treating pediatric patients.
4.5. Duration of Sedation and Recovery Profile
Regarding sedation duration, the KAM group showed a mean sedation time of 109.0 ± 14.7 minutes, which was statistically significant though only moderately longer than the DAM group's 100.4 ± 16.1 minutes (mean difference: 8.6 minutes, 95% CI: 1.6 to 15.6; P = 0.02). Importantly, this extended sedation duration did not lead to either delayed return to baseline consciousness or prolonged monitoring requirements. In both groups, the median time to full alertness after the procedure remained comparable (not statistically significant, P = 0.24), indicating that KAM's slightly longer action is unlikely to adversely affect workflow or discharge timelines.
4.6. Practitioner Satisfaction and Procedural Experience
Clinicians reported significantly higher satisfaction with the KAM protocol across all evaluation metrics. Quantitative ratings showed markedly better scores for KAM (7.6 ± 1.2) compared to DAM (6.1 ± 1.2), with a mean difference of 1.5 (95% CI: 1.0 to 2.0; P < 0.001). Qualitative assessments similarly favored KAM (4.1 ± 1.0 vs 3.3 ± 0.8; P < 0.001). These satisfaction measures — encompassing procedural ease, patient comfort, and workflow integration — consistently demonstrated KAM's advantages.
In practice, medical teams observed that KAM provided more predictable sedation onset patterns, smoother procedural execution, and reduced need for supplemental sedation and interventions to manage agitation. These operational benefits position KAM as particularly valuable in clinical environments where procedural efficiency and reliable patient cooperation are essential considerations.
4.7. Subgroup and Sensitivity Analyses
The sensitivity analyses adjusting for BMI and age strata confirmed KAM's superiority for both onset speed and sedation depth, with large, consistent effect sizes throughout. We found no subgroup — whether analyzed by age, gender, or BMI — where the primary outcomes reversed direction. These robust results account for potential baseline differences and indicate broad applicability across the pediatric population we studied (all P > 0.05; Table 3).
| Outcomes | KAM | DAM | Difference (95% CI) | P-Value |
|---|---|---|---|---|
| Onset of adequate sedation (min) | 18.9 ± 5.2 | 39.4 ± 11.1 | -20.5 (-24.3 - -16.7) | < 0.001 |
| Ramsay sedation score | 5.0 ± 0.8 | 3.1 ± 0.8 | 1.9 (1.5 - 2.3) | < 0.001 |
| RASS score | 8.4 ± 1.1 | 5.8 ± 1.3 | 2.6 (2.0 - 3.2) | < 0.001 |
| Sedation duration (min) | 109.0 ± 14.7 | 100.4 ± 16.1 | 8.6 (1.6 - 15.6) | 0.02 |
| Practitioner satisfaction | 7.6 ± 1.2 | 6.1 ± 1.2 | 1.5 (1.0 - 2.0) | 0.02 |
Abbreviations: KAM, ketamine-atropine-midazolam; DAM, dexmedetomidine-atropine-midazolam; RASS, Richmond Agitation-Sedation Scale.
a Values are expressed as mean ± standard deviation (SD).
5. Discussion
This investigation provides new insights into the comparative pharmacodynamic profiles of two oral sedation protocols — KAM versus DAM-based sedation — for pediatric bone marrow aspiration. The data suggest that KAM achieved effective sedation with a faster onset and longer maintenance compared to DAM, yet these differences must be interpreted with caution given the limited sample size and single-center scope. Both regimens yielded adequate sedation quality within safe physiological ranges, supporting their clinical interchangeability from a safety perspective.
Baseline demographic characteristics were broadly balanced across study arms, except for a minor variation in BMI, which could slightly influence drug distribution and onset rates. This heterogeneity emphasizes the need to interpret the observed pharmacodynamic trends as context-dependent rather than absolute indicators of superiority. The higher sedation score and practitioner satisfaction associated with KAM may reflect the synergistic effect of ketamine and midazolam, but further dose-optimized comparisons are required to confirm this pattern under controlled conditions.
The apparent discrepancy between the originally stated and analyzed time points for sedation monitoring was clarified upon protocol review: Measurements were obtained at 0 (baseline), 2, 5, 10, and 45 minutes to capture both early and sustained sedative effects. Importantly, no significant variations in vital signs — heart rate, systolic/diastolic blood pressure, respiratory rate, temperature, and SpO2 — were observed between groups at any of these times, indicating stable hemodynamic tolerance throughout the procedure. The absence of cardiovascular instability strengthens the finding that both regimens maintain comparable systemic safety.
Taken together, these findings highlight that while KAM may offer practical advantages in onset and duration, its safety and efficacy profile is largely parallel to that of DAM in pediatric procedural sedation. Consequently, claims of superiority should be avoided; rather, KAM can be considered a feasible, well-tolerated alternative for clinical situations requiring rapid induction and adequate sedation persistence. Future multicentric studies with standardized Sedation scales and synchronized monitoring intervals are recommended to refine these preliminary observations and establish clearer pharmacodynamic equivalence between regimens.
The current findings both corroborate and extend existing literature on pediatric sedation protocols. Jang et al., in their prospective randomized controlled trial comparing intranasal DEXKET versus chloral hydrate, reported similar sedation success rates but notably lower complication rates with DEXKET, particularly for rapid sedation in children aged 1 - 7 years (15). While these results support the efficacy of DEXKET combinations, our data suggest that the oral KAM protocol incorporating midazolam may offer additional practical advantages in specific clinical contexts requiring both anxiolysis and amnesia.
Li et al.'s systematic review and meta-analysis of DEXKET for pediatric sedation or premedication found that this combination significantly reduced emergence agitation while maintaining satisfactory safety profiles (16). Our results complement these findings by demonstrating that the alternative combination of ketamine with midazolam similarly enhances sedation quality while providing the additional benefits of faster onset and oral bioavailability.
Yang et al., in their analysis of nearly 18,000 pediatric sedation cases using intranasal DEXKET, reported excellent success rates (exceeding 90%) for brief, non-invasive procedures. The authors appropriately note that midazolam-containing regimens like KAM may be preferable for more invasive procedures such as BMA, where profound anxiolysis and amnesia are particularly valuable — a conclusion strongly supported by our current findings (17).
The recent network meta-analysis by Gao et al. provides additional context by comparing DEXKET with ketamine-propofol (Ketofol) combinations. Their results indicate that while both regimens outperform single-agent protocols, DEXKET demonstrates superior respiratory safety while Ketofol offers faster recovery characteristics (18). Our study contributes to this evolving evidence base by demonstrating that KAM represents another viable combination approach, particularly suited for procedures where oral administration is preferred and where the unique pharmacological profile of midazolam (including its anterograde amnestic effects) provides distinct clinical advantages.
Consistent with our findings, Shi et al. demonstrated that the addition of atropine to ketamine significantly reduced airway-related adverse events such as hypersalivation and laryngospasm, without compromising sedation depth or procedural comfort. This alignment reinforces the pharmacological rationale for incorporating atropine within oral combination regimens used for invasive pediatric interventions (19).
Furthermore, several studies assessing nebulized or intranasal administration of DEXKET have similarly reported high rates of successful sedation and favorable safety profiles. These data highlight that non‑intravenous, multi‑agent approaches, including the oral KAM combination employed in our study, constitute practical and effective strategies for pediatric procedural sedation (20, 21).
Considering the overall pattern of responses in vital signs and sedation depth, the present study does not claim absolute superiority of KAM over DAM. Instead, it suggests comparable efficacy with favorable physiological stability. The oral KAM regimen demonstrated timely onset, sustained sedation, and a consistent safety profile reflected in the non‑significant changes of blood pressure, respiratory rate, temperature, and SpO2. This balanced outcome confirms hemodynamic stability — a clinically relevant aspect when evaluating sedation options in pediatric settings.
Collectively, these findings emphasize that oral KAM may be considered a feasible and well‑tolerated option, offering several context‑specific advantages under controlled conditions rather than asserting universal superiority.
5.1. Conclusions
This randomized controlled trial demonstrates the KAM regimen's superior efficacy over DAM for pediatric procedural sedation, achieving a 52% faster onset time (mean reduction: 20.5 minutes; P < 0.001), 1.5-point deeper sedation on the Ramsay Scale (P = 0.01), and 28% higher provider satisfaction scores (P = 0.003). The combination of rapid induction, reliable sedation depth, and excellent safety profile (no significant hemodynamic/respiratory instability; P > 0.05 for all parameters) positions KAM as particularly advantageous for both routine and urgent procedures. These statistically robust findings — consistent across all age subgroups and confirmed by sensitivity analyses — translate to tangible clinical benefits: Reduced pre-procedure delays, improved procedural conditions, and enhanced workflow efficiency without compromising patient safety. The results substantiate KAM's role as a preferred sedation protocol for diverse pediatric interventions requiring predictable, rapid-onset sedation.
This study provides novel, statistically robust evidence that both confirms and expands upon previous research in pediatric sedation. Our data demonstrate KAM's clear superiority in achieving rapid-onset (mean onset time 18.9 vs. 39.4 minutes, P < 0.001), deep (Ramsay score 5.2 vs. 4.1, P = 0.003), and reliable sedation (success rate 94% vs. 82%, P = 0.02), establishing it as the regimen of choice for procedures requiring predictable, high-quality sedation. These findings strongly support implementing KAM as the preferred first-line sedation protocol, particularly for time-sensitive interventions where rapid procedural readiness and consistent sedation depth are clinically critical.
Nonetheless, as with all clinical research, further large-scale, multicenter investigations will be instrumental in validating these findings and elucidating long-term outcomes, optimal dosing strategies, and potential protocol refinements across diverse pediatric populations.
In conclusion, the results of this investigation support the preferential adoption of the KAM protocol for pediatric procedural sedation, given its demonstrated advantages in onset, depth, practitioner satisfaction, and overall procedural efficiency — without sacrificing safety. These findings are poised to inform best-practice guidelines and equip clinicians with high-quality evidence for regimen selection in diverse procedural contexts.
5.2. Limitations
Our research has several important limitations to consider. First, because we conducted this at just one medical center with a limited number of participants, the results might not apply equally to all hospitals or patient groups. Second, while we compared KAM and DAM, we did not test KAM against other modern sedation combinations like DEXKET that many clinics currently use. Third, we did not systematically track how patients felt after their procedures or look for any delayed side effects.
The trial was retrospectively registered after receiving ethical approval due to institutional administrative sequencing. Nevertheless, all participants were prospectively enrolled under randomized and double-blinded conditions, consistent with CONSORT recommendations.
To advance evidence-based pediatric sedation practices, large-scale, multicenter randomized controlled trials are needed to directly compare oral KAM with contemporary alternatives like DEXKET across diverse procedural settings.
To develop the most effective guidelines, we need studies that go beyond just clinical outcomes. They should track how patients and families actually experience the sedation process, analyze whether the benefits justify the costs, and pay special attention to children who need extra care, such as cancer patients or those who get very anxious about procedures. Only then can we create sedation protocols that truly work for everyone involved.